 Found 32 hits of Enzyme Inhibition Constant Data
Found 32 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331593

(3-(2-morpholino-6-(pyridin-3-yloxy)pyrimidin-4-yl)...)Show InChI InChI=1S/C19H18N4O3/c24-15-4-1-3-14(11-15)17-12-18(26-16-5-2-6-20-13-16)22-19(21-17)23-7-9-25-10-8-23/h1-6,11-13,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331591
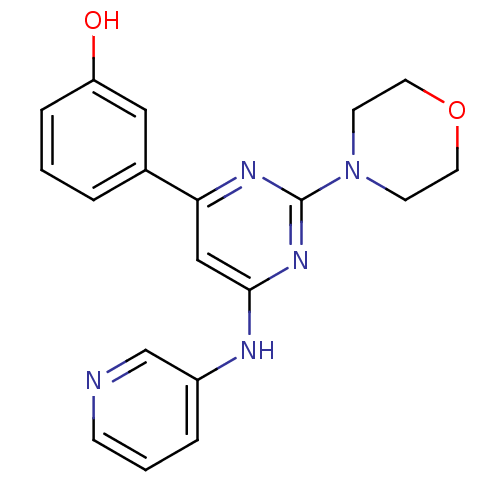
(3-(2-morpholino-6-(pyridin-3-ylamino)pyrimidin-4-y...)Show InChI InChI=1S/C19H19N5O2/c25-16-5-1-3-14(11-16)17-12-18(21-15-4-2-6-20-13-15)23-19(22-17)24-7-9-26-10-8-24/h1-6,11-13,25H,7-10H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331590

(3-(6-(4-methoxyphenylamino)-2-morpholinopyrimidin-...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cc1 Show InChI InChI=1S/C21H22N4O3/c1-27-18-7-5-16(6-8-18)22-20-14-19(15-3-2-4-17(26)13-15)23-21(24-20)25-9-11-28-12-10-25/h2-8,13-14,26H,9-12H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331587
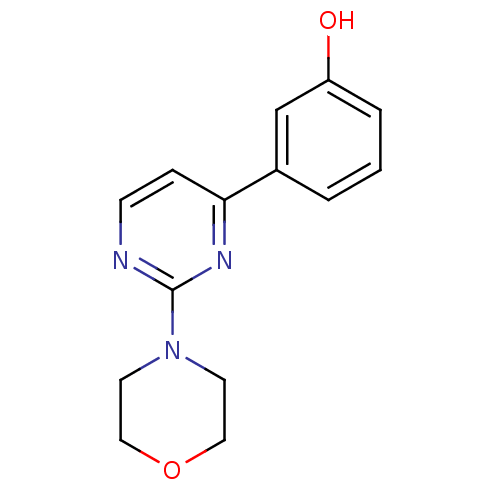
(3-(2-morpholinopyrimidin-4-yl)phenol | CHEMBL12886...)Show InChI InChI=1S/C14H15N3O2/c18-12-3-1-2-11(10-12)13-4-5-15-14(16-13)17-6-8-19-9-7-17/h1-5,10,18H,6-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331595

(3-(6-(4-hydroxycyclohexylamino)-2-morpholinopyrimi...)Show SMILES OC1CCC(CC1)Nc1cc(nc(n1)N1CCOCC1)-c1cccc(O)c1 |(29.43,-18.98,;28.1,-19.75,;26.75,-18.99,;25.43,-19.77,;25.44,-21.3,;26.77,-22.07,;28.1,-21.29,;24.1,-22.07,;24.11,-23.61,;22.78,-24.39,;22.78,-25.93,;24.11,-26.7,;25.45,-25.93,;25.45,-24.38,;26.78,-26.7,;26.78,-28.24,;28.11,-29.01,;29.45,-28.24,;29.45,-26.7,;28.11,-25.92,;21.45,-26.7,;20.11,-25.93,;18.78,-26.69,;18.77,-28.24,;20.12,-29.01,;20.12,-30.55,;21.45,-28.24,)| Show InChI InChI=1S/C20H26N4O3/c25-16-6-4-15(5-7-16)21-19-13-18(14-2-1-3-17(26)12-14)22-20(23-19)24-8-10-27-11-9-24/h1-3,12-13,15-16,25-26H,4-11H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331597

(3-(2,6-dimorpholinopyrimidin-4-yl)phenol | CHEMBL1...)Show InChI InChI=1S/C18H22N4O3/c23-15-3-1-2-14(12-15)16-13-17(21-4-8-24-9-5-21)20-18(19-16)22-6-10-25-11-7-22/h1-3,12-13,23H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331596
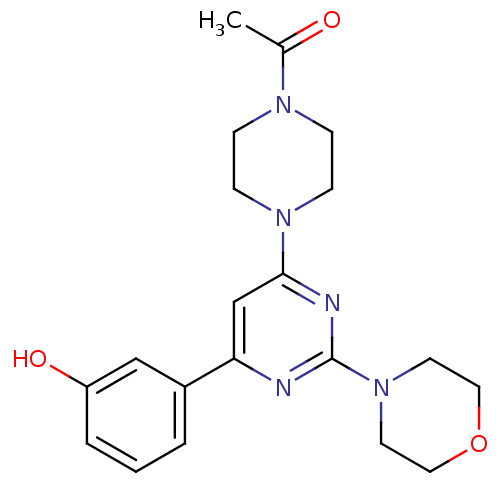
(1-(4-(6-(3-hydroxyphenyl)-2-morpholinopyrimidin-4-...)Show SMILES CC(=O)N1CCN(CC1)c1cc(nc(n1)N1CCOCC1)-c1cccc(O)c1 Show InChI InChI=1S/C20H25N5O3/c1-15(26)23-5-7-24(8-6-23)19-14-18(16-3-2-4-17(27)13-16)21-20(22-19)25-9-11-28-12-10-25/h2-4,13-14,27H,5-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331578

(2-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1ccccc1-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-19-4-2-1-3-16(19)18-12-20(25-21(24-18)27-7-9-29-10-8-27)23-15-5-6-17-14(11-15)13-22-26-17/h1-6,11-13,28H,7-10H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331588

(3-(6-(methylamino)-2-morpholinopyrimidin-4-yl)phen...)Show InChI InChI=1S/C15H18N4O2/c1-16-14-10-13(11-3-2-4-12(20)9-11)17-15(18-14)19-5-7-21-8-6-19/h2-4,9-10,20H,5-8H2,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331589

(3-(2-morpholino-6-(phenylamino)pyrimidin-4-yl)phen...)Show InChI InChI=1S/C20H20N4O2/c25-17-8-4-5-15(13-17)18-14-19(21-16-6-2-1-3-7-16)23-20(22-18)24-9-11-26-12-10-24/h1-8,13-14,25H,9-12H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331594
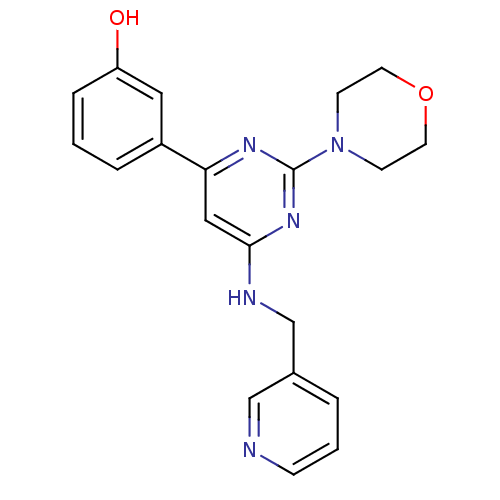
(3-(2-morpholino-6-(pyridin-3-ylmethylamino)pyrimid...)Show InChI InChI=1S/C20H21N5O2/c26-17-5-1-4-16(11-17)18-12-19(22-14-15-3-2-6-21-13-15)24-20(23-18)25-7-9-27-10-8-25/h1-6,11-13,26H,7-10,14H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331598
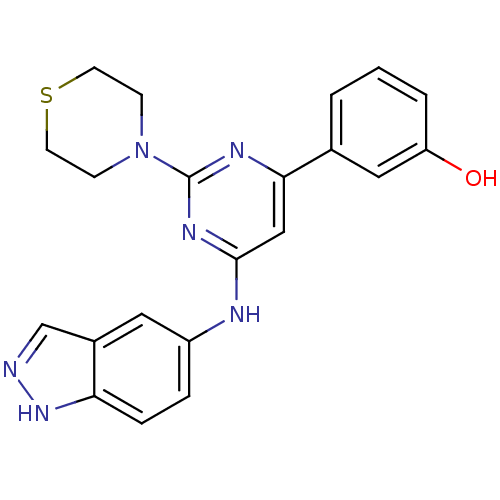
(3-(6-(1H-indazol-5-ylamino)-2-thiomorpholinopyrimi...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCSCC1 Show InChI InChI=1S/C21H20N6OS/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331579

(4-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1ccc(cc1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-4-1-14(2-5-17)19-12-20(25-21(24-19)27-7-9-29-10-8-27)23-16-3-6-18-15(11-16)13-22-26-18/h1-6,11-13,28H,7-10H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331599
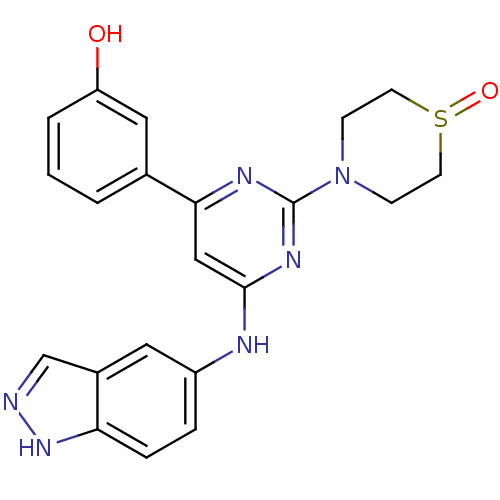
(3-[6-(1H-Indazol-5-ylamino)-2-(1-oxo-1lambda4-thio...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCS(=O)CC1 Show InChI InChI=1S/C21H20N6O2S/c28-17-3-1-2-14(11-17)19-12-20(23-16-4-5-18-15(10-16)13-22-26-18)25-21(24-19)27-6-8-30(29)9-7-27/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331580
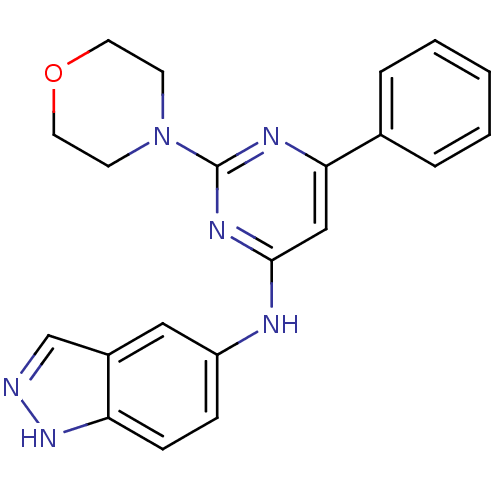
(CHEMBL1290175 | N-(2-morpholino-6-phenylpyrimidin-...)Show SMILES C1CN(CCO1)c1nc(Nc2ccc3[nH]ncc3c2)cc(n1)-c1ccccc1 Show InChI InChI=1S/C21H20N6O/c1-2-4-15(5-3-1)19-13-20(25-21(24-19)27-8-10-28-11-9-27)23-17-6-7-18-16(12-17)14-22-26-18/h1-7,12-14H,8-11H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331581
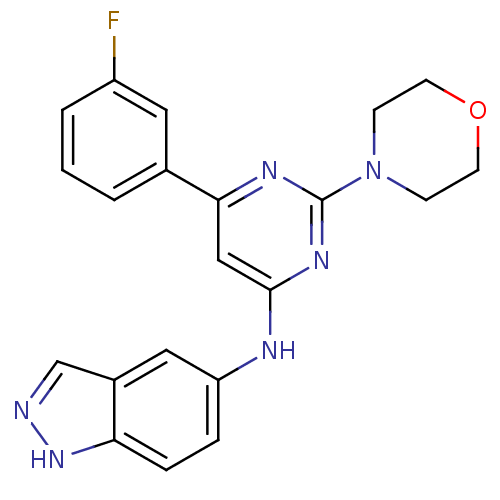
(CHEMBL1290176 | N-(6-(3-fluorophenyl)-2-morpholino...)Show SMILES Fc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H19FN6O/c22-16-3-1-2-14(10-16)19-12-20(26-21(25-19)28-6-8-29-9-7-28)24-17-4-5-18-15(11-17)13-23-27-18/h1-5,10-13H,6-9H2,(H,23,27)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331586

(2-(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimid...)Show SMILES OCCOc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H24N6O3/c30-8-11-32-19-3-1-2-16(13-19)21-14-22(27-23(26-21)29-6-9-31-10-7-29)25-18-4-5-20-17(12-18)15-24-28-20/h1-5,12-15,30H,6-11H2,(H,24,28)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331602
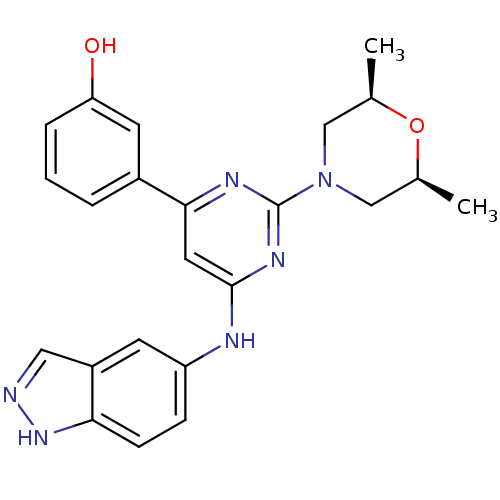
(3-(6-(1H-indazol-5-ylamino)-2-((2S,6R)-2,6-dimethy...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1nc(Nc2ccc3[nH]ncc3c2)cc(n1)-c1cccc(O)c1 |r| Show InChI InChI=1S/C23H24N6O2/c1-14-12-29(13-15(2)31-14)23-26-21(16-4-3-5-19(30)9-16)10-22(27-23)25-18-6-7-20-17(8-18)11-24-28-20/h3-11,14-15,30H,12-13H2,1-2H3,(H,24,28)(H,25,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331582
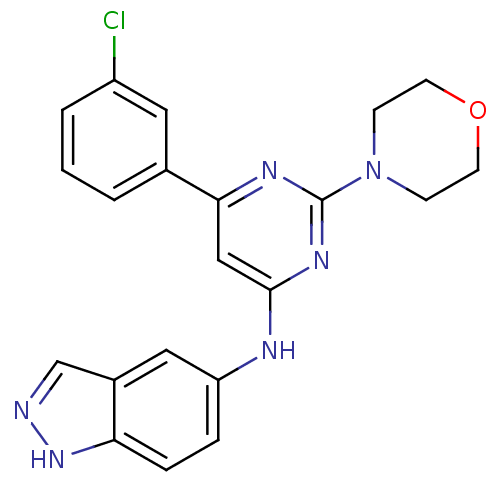
(CHEMBL1290286 | N-(6-(3-chlorophenyl)-2-morpholino...)Show SMILES Clc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H19ClN6O/c22-16-3-1-2-14(10-16)19-12-20(26-21(25-19)28-6-8-29-9-7-28)24-17-4-5-18-15(11-17)13-23-27-18/h1-5,10-13H,6-9H2,(H,23,27)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331584

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES N#Cc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H19N7O/c23-13-15-2-1-3-16(10-15)20-12-21(27-22(26-20)29-6-8-30-9-7-29)25-18-4-5-19-17(11-18)14-24-28-19/h1-5,10-12,14H,6-9H2,(H,24,28)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331601

(3-(6-(1H-indazol-5-ylamino)-2-(2-methoxyethylamino...)Show SMILES COCCNc1nc(Nc2ccc3[nH]ncc3c2)cc(n1)-c1cccc(O)c1 Show InChI InChI=1S/C20H20N6O2/c1-28-8-7-21-20-24-18(13-3-2-4-16(27)10-13)11-19(25-20)23-15-5-6-17-14(9-15)12-22-26-17/h2-6,9-12,27H,7-8H2,1H3,(H,22,26)(H2,21,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331583

(CHEMBL1290287 | N-(6-(3-methoxyphenyl)-2-morpholin...)Show SMILES COc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H22N6O2/c1-29-18-4-2-3-15(12-18)20-13-21(26-22(25-20)28-7-9-30-10-8-28)24-17-5-6-19-16(11-17)14-23-27-19/h2-6,11-14H,7-10H2,1H3,(H,23,27)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331585
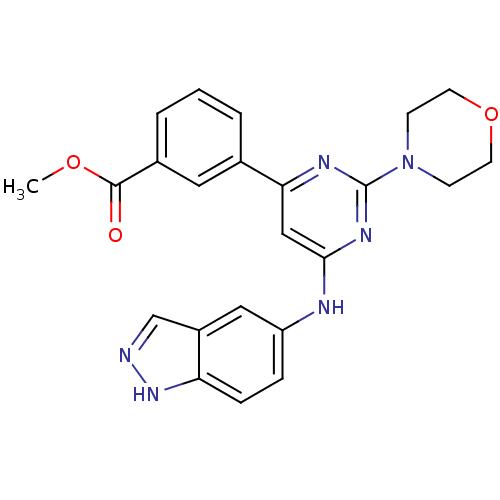
(CHEMBL1290403 | methyl 3-(6-(1H-indazol-5-ylamino)...)Show SMILES COC(=O)c1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H22N6O3/c1-31-22(30)16-4-2-3-15(11-16)20-13-21(27-23(26-20)29-7-9-32-10-8-29)25-18-5-6-19-17(12-18)14-24-28-19/h2-6,11-14H,7-10H2,1H3,(H,24,28)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331600

(3-(6-(1H-indazol-5-ylamino)-2-(piperazin-1-yl)pyri...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCNCC1 Show InChI InChI=1S/C21H21N7O/c29-17-3-1-2-14(11-17)19-12-20(26-21(25-19)28-8-6-22-7-9-28)24-16-4-5-18-15(10-16)13-23-27-18/h1-5,10-13,22,29H,6-9H2,(H,23,27)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data