 Found 33 hits of Enzyme Inhibition Constant Data
Found 33 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50333880
(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of rat liver cytosolic TrxR1 by Lineweaver-Burk plot analysis |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of rat liver cytosolic TrxR1 at protein-substrate complex by Lineweaver-Burk plot analysis |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Glutathione reductase
(Saccharomyces cerevisiae) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of yeast glutathione reductase by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Glutathione reductase
(Saccharomyces cerevisiae) | BDBM50060874

(1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...)Show SMILES Cc1cc(O)c2c3c1c1c(C)cc(O)c4c1c1c3c3c(c(O)cc(O)c3c2=O)c2c(O)cc(O)c(c12)c4=O Show InChI InChI=1S/C30H16O8/c1-7-3-9(31)19-23-15(7)16-8(2)4-10(32)20-24(16)28-26-18(12(34)6-14(36)22(26)30(20)38)17-11(33)5-13(35)21(29(19)37)25(17)27(23)28/h3-6,31-36H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of yeast glutathione reductase by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50060874

(1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...)Show SMILES Cc1cc(O)c2c3c1c1c(C)cc(O)c4c1c1c3c3c(c(O)cc(O)c3c2=O)c2c(O)cc(O)c(c12)c4=O Show InChI InChI=1S/C30H16O8/c1-7-3-9(31)19-23-15(7)16-8(2)4-10(32)20-24(16)28-26-18(12(34)6-14(36)22(26)30(20)38)17-11(33)5-13(35)21(29(19)37)25(17)27(23)28/h3-6,31-36H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50060874

(1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...)Show SMILES Cc1cc(O)c2c3c1c1c(C)cc(O)c4c1c1c3c3c(c(O)cc(O)c3c2=O)c2c(O)cc(O)c(c12)c4=O Show InChI InChI=1S/C30H16O8/c1-7-3-9(31)19-23-15(7)16-8(2)4-10(32)20-24(16)28-26-18(12(34)6-14(36)22(26)30(20)38)17-11(33)5-13(35)21(29(19)37)25(17)27(23)28/h3-6,31-36H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM32021
(4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracen...)Show InChI InChI=1S/C15H8O6/c16-9-3-1-2-7-11(9)14(19)12-8(13(7)18)4-6(15(20)21)5-10(12)17/h1-5,16-17H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM32021

(4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracen...)Show InChI InChI=1S/C15H8O6/c16-9-3-1-2-7-11(9)14(19)12-8(13(7)18)4-6(15(20)21)5-10(12)17/h1-5,16-17H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50005886
(1,8-Dihydroxy-3-methoxy-6-methylanthraquinone | 1,...)Show InChI InChI=1S/C16H12O5/c1-7-3-9-13(11(17)4-7)16(20)14-10(15(9)19)5-8(21-2)6-12(14)18/h3-6,17-18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50085551
(1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...)Show InChI InChI=1S/C15H10O5/c16-6-7-4-9-13(11(18)5-7)15(20)12-8(14(9)19)2-1-3-10(12)17/h1-5,16-18H,6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50455992
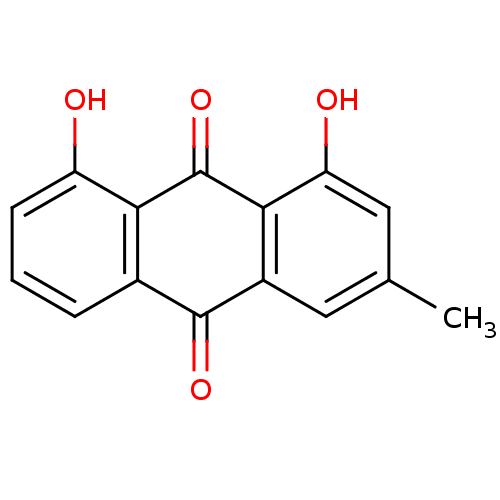
(CHEBI:3687 | Chrysophanol)Show InChI InChI=1S/C15H10O4/c1-7-5-9-13(11(17)6-7)15(19)12-8(14(9)18)3-2-4-10(12)16/h2-6,16-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50005886

(1,8-Dihydroxy-3-methoxy-6-methylanthraquinone | 1,...)Show InChI InChI=1S/C16H12O5/c1-7-3-9-13(11(17)4-7)16(20)14-10(15(9)19)5-8(21-2)6-12(14)18/h3-6,17-18H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50455992

(CHEBI:3687 | Chrysophanol)Show InChI InChI=1S/C15H10O4/c1-7-5-9-13(11(17)6-7)15(19)12-8(14(9)18)3-2-4-10(12)16/h2-6,16-17H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50060874

(1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...)Show SMILES Cc1cc(O)c2c3c1c1c(C)cc(O)c4c1c1c3c3c(c(O)cc(O)c3c2=O)c2c(O)cc(O)c(c12)c4=O Show InChI InChI=1S/C30H16O8/c1-7-3-9(31)19-23-15(7)16-8(2)4-10(32)20-24(16)28-26-18(12(34)6-14(36)22(26)30(20)38)17-11(33)5-13(35)21(29(19)37)25(17)27(23)28/h3-6,31-36H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50060874

(1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...)Show SMILES Cc1cc(O)c2c3c1c1c(C)cc(O)c4c1c1c3c3c(c(O)cc(O)c3c2=O)c2c(O)cc(O)c(c12)c4=O Show InChI InChI=1S/C30H16O8/c1-7-3-9(31)19-23-15(7)16-8(2)4-10(32)20-24(16)28-26-18(12(34)6-14(36)22(26)30(20)38)17-11(33)5-13(35)21(29(19)37)25(17)27(23)28/h3-6,31-36H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50240382
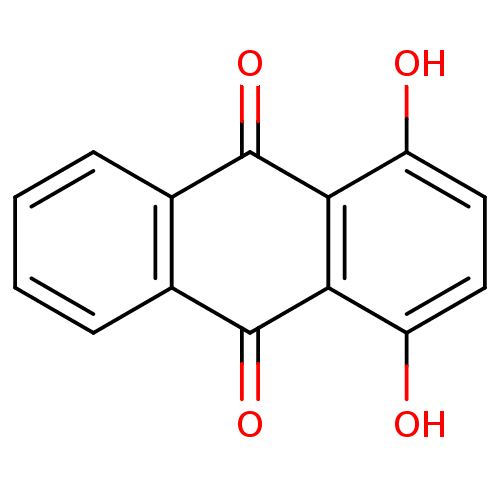
(1,4-Dihydroxyanthrachinon | 1,4-dihydroxy-9,10-ant...)Show InChI InChI=1S/C14H8O4/c15-9-5-6-10(16)12-11(9)13(17)7-3-1-2-4-8(7)14(12)18/h1-6,15-16H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50060860

(9(10H)-anthracenone | 9,10-dihydro-9-oxoanthracene...)Show InChI InChI=1S/C14H10O/c15-14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50240382

(1,4-Dihydroxyanthrachinon | 1,4-dihydroxy-9,10-ant...)Show InChI InChI=1S/C14H8O4/c15-9-5-6-10(16)12-11(9)13(17)7-3-1-2-4-8(7)14(12)18/h1-6,15-16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50060860

(9(10H)-anthracenone | 9,10-dihydro-9-oxoanthracene...)Show InChI InChI=1S/C14H10O/c15-14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-8H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50333878

(CHEMBL1172006 | Frangulin A)Show SMILES C[C@@H]1O[C@@H](Oc2cc(O)c3C(=O)c4c(O)cc(C)cc4C(=O)c3c2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20O9/c1-7-3-10-14(12(22)4-7)18(26)15-11(17(10)25)5-9(6-13(15)23)30-21-20(28)19(27)16(24)8(2)29-21/h3-6,8,16,19-24,27-28H,1-2H3/t8-,16-,19+,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50085551

(1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...)Show InChI InChI=1S/C15H10O5/c16-6-7-4-9-13(11(18)5-7)15(20)12-8(14(9)19)2-1-3-10(12)17/h1-5,16-18H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50333878

(CHEMBL1172006 | Frangulin A)Show SMILES C[C@@H]1O[C@@H](Oc2cc(O)c3C(=O)c4c(O)cc(C)cc4C(=O)c3c2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20O9/c1-7-3-10-14(12(22)4-7)18(26)15-11(17(10)25)5-9(6-13(15)23)30-21-20(28)19(27)16(24)8(2)29-21/h3-6,8,16,19-24,27-28H,1-2H3/t8-,16-,19+,20+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM11316
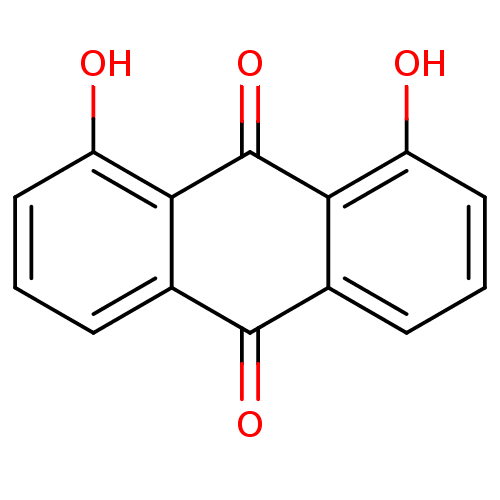
(1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...)Show InChI InChI=1S/C14H8O4/c15-9-5-1-3-7-11(9)14(18)12-8(13(7)17)4-2-6-10(12)16/h1-6,15-16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM11316

(1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...)Show InChI InChI=1S/C14H8O4/c15-9-5-1-3-7-11(9)14(18)12-8(13(7)17)4-2-6-10(12)16/h1-6,15-16H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM80739
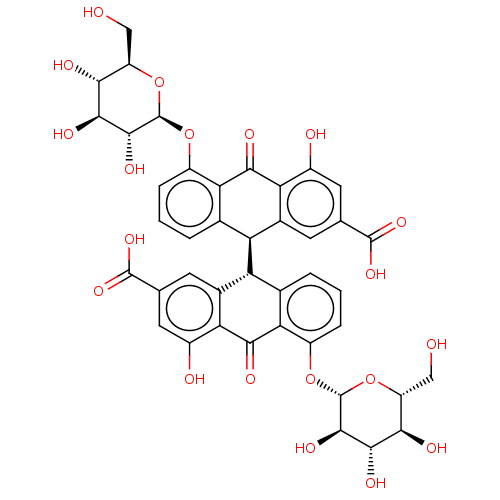
(MLS002473001 | SMR001397106 | cid_91440 | sennosid...)Show SMILES [H][C@@]1(c2cccc(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2C(=O)c2c(O)cc(cc12)C(O)=O)[C@]1([H])c2cccc(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2C(=O)c2c(O)cc(cc12)C(O)=O Show InChI InChI=1S/C42H38O20/c43-11-23-31(47)35(51)37(53)41(61-23)59-21-5-1-3-15-25(17-7-13(39(55)56)9-19(45)27(17)33(49)29(15)21)26-16-4-2-6-22(60-42-38(54)36(52)32(48)24(12-44)62-42)30(16)34(50)28-18(26)8-14(40(57)58)10-20(28)46/h1-10,23-26,31-32,35-38,41-48,51-54H,11-12H2,(H,55,56)(H,57,58)/t23-,24-,25-,26+,31-,32-,35+,36+,37-,38-,41-,42-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50333879
(CHEMBL1644068 | Skyrin glucoside)Show SMILES Cc1cc(O)c2C(=O)c3c(O)cc(O)c(c3C(=O)c2c1)-c1c(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c2C(=O)c3c(O)cc(C)cc3C(=O)c12 |r,wU:30.33,25.28,23.25,wD:32.36,28.31,(-.47,-40.27,;.87,-41.03,;.87,-42.57,;2.19,-43.34,;2.19,-44.88,;3.52,-42.57,;4.84,-43.34,;4.84,-44.88,;6.15,-42.58,;7.48,-43.37,;7.48,-44.91,;8.83,-42.59,;8.83,-41.05,;10.27,-40.52,;7.49,-40.3,;6.16,-41.04,;4.84,-40.28,;4.84,-38.74,;3.51,-41.04,;2.19,-40.29,;7.54,-38.01,;6.21,-37.25,;4.87,-38.01,;3.54,-37.25,;3.54,-35.71,;2.21,-34.93,;2.21,-33.39,;.88,-32.62,;.88,-35.7,;-.47,-34.91,;.87,-37.24,;-.46,-38,;2.2,-38.01,;2.2,-39.55,;6.21,-35.71,;7.54,-34.93,;7.54,-33.39,;8.88,-35.7,;10.21,-34.9,;10.21,-33.36,;11.56,-35.69,;12.9,-34.9,;12.9,-33.36,;14.25,-35.67,;14.26,-37.22,;15.6,-37.99,;12.92,-38,;11.57,-37.24,;10.22,-38.02,;10.22,-39.56,;8.88,-37.25,)| Show InChI InChI=1S/C36H28O15/c1-10-3-12-21(14(38)5-10)32(46)24-17(41)7-16(40)23(27(24)29(12)43)26-19(50-36-35(49)34(48)31(45)20(9-37)51-36)8-18(42)25-28(26)30(44)13-4-11(2)6-15(39)22(13)33(25)47/h3-8,20,31,34-42,45,48-49H,9H2,1-2H3/t20-,31-,34+,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50333879

(CHEMBL1644068 | Skyrin glucoside)Show SMILES Cc1cc(O)c2C(=O)c3c(O)cc(O)c(c3C(=O)c2c1)-c1c(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c2C(=O)c3c(O)cc(C)cc3C(=O)c12 |r,wU:30.33,25.28,23.25,wD:32.36,28.31,(-.47,-40.27,;.87,-41.03,;.87,-42.57,;2.19,-43.34,;2.19,-44.88,;3.52,-42.57,;4.84,-43.34,;4.84,-44.88,;6.15,-42.58,;7.48,-43.37,;7.48,-44.91,;8.83,-42.59,;8.83,-41.05,;10.27,-40.52,;7.49,-40.3,;6.16,-41.04,;4.84,-40.28,;4.84,-38.74,;3.51,-41.04,;2.19,-40.29,;7.54,-38.01,;6.21,-37.25,;4.87,-38.01,;3.54,-37.25,;3.54,-35.71,;2.21,-34.93,;2.21,-33.39,;.88,-32.62,;.88,-35.7,;-.47,-34.91,;.87,-37.24,;-.46,-38,;2.2,-38.01,;2.2,-39.55,;6.21,-35.71,;7.54,-34.93,;7.54,-33.39,;8.88,-35.7,;10.21,-34.9,;10.21,-33.36,;11.56,-35.69,;12.9,-34.9,;12.9,-33.36,;14.25,-35.67,;14.26,-37.22,;15.6,-37.99,;12.92,-38,;11.57,-37.24,;10.22,-38.02,;10.22,-39.56,;8.88,-37.25,)| Show InChI InChI=1S/C36H28O15/c1-10-3-12-21(14(38)5-10)32(46)24-17(41)7-16(40)23(27(24)29(12)43)26-19(50-36-35(49)34(48)31(45)20(9-37)51-36)8-18(42)25-28(26)30(44)13-4-11(2)6-15(39)22(13)33(25)47/h3-8,20,31,34-42,45,48-49H,9H2,1-2H3/t20-,31-,34+,35-,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM50269016
(CHEMBL497001 | aloin)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1c2cccc(O)c2C(=O)c2c(O)cc(CO)cc12 |r| Show InChI InChI=1S/C21H22O9/c22-6-8-4-10-14(21-20(29)19(28)17(26)13(7-23)30-21)9-2-1-3-11(24)15(9)18(27)16(10)12(25)5-8/h1-5,13-14,17,19-26,28-29H,6-7H2/t13-,14?,17-,19+,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 2, mitochondrial
(Rattus norvegicus) | BDBM80739

(MLS002473001 | SMR001397106 | cid_91440 | sennosid...)Show SMILES [H][C@@]1(c2cccc(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2C(=O)c2c(O)cc(cc12)C(O)=O)[C@]1([H])c2cccc(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2C(=O)c2c(O)cc(cc12)C(O)=O Show InChI InChI=1S/C42H38O20/c43-11-23-31(47)35(51)37(53)41(61-23)59-21-5-1-3-15-25(17-7-13(39(55)56)9-19(45)27(17)33(49)29(15)21)26-16-4-2-6-22(60-42-38(54)36(52)32(48)24(12-44)62-42)30(16)34(50)28-18(26)8-14(40(57)58)10-20(28)46/h1-10,23-26,31-32,35-38,41-48,51-54H,11-12H2,(H,55,56)(H,57,58)/t23-,24-,25-,26+,31-,32-,35+,36+,37-,38-,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Rattus norvegicus) | BDBM50269016

(CHEMBL497001 | aloin)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1c2cccc(O)c2C(=O)c2c(O)cc(CO)cc12 |r| Show InChI InChI=1S/C21H22O9/c22-6-8-4-10-14(21-20(29)19(28)17(26)13(7-23)30-21)9-2-1-3-11(24)15(9)18(27)16(10)12(25)5-8/h1-5,13-14,17,19-26,28-29H,6-7H2/t13-,14?,17-,19+,20-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of rat liver cytosolic TrxR1 by spectrophotometry |
Bioorg Med Chem 19: 631-41 (2011)
Article DOI: 10.1016/j.bmc.2010.10.045
BindingDB Entry DOI: 10.7270/Q25D8S4N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data