 Found 26 hits of Enzyme Inhibition Constant Data
Found 26 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333703
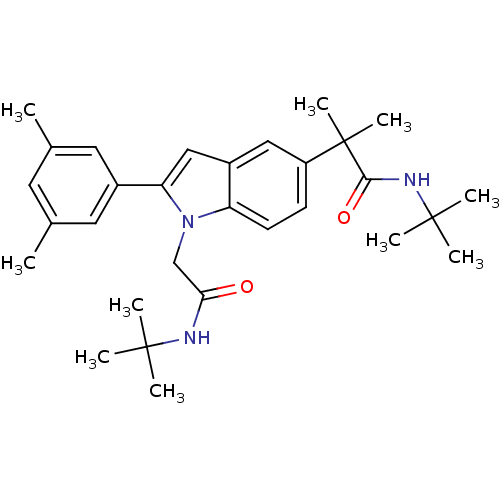
(CHEMBL1643724 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES Cc1cc(C)cc(c1)-c1cc2cc(ccc2n1CC(=O)NC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H41N3O2/c1-19-13-20(2)15-21(14-19)25-17-22-16-23(30(9,10)27(35)32-29(6,7)8)11-12-24(22)33(25)18-26(34)31-28(3,4)5/h11-17H,18H2,1-10H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333709
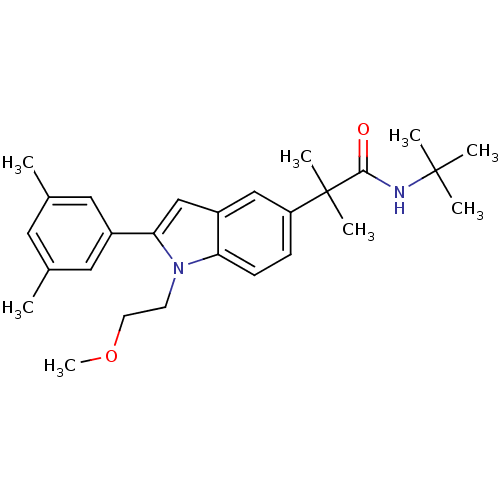
(CHEMBL1643725 | N-tert-butyl-2-(2-(3,5-dimethylphe...)Show SMILES COCCn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C27H36N2O2/c1-18-13-19(2)15-20(14-18)24-17-21-16-22(9-10-23(21)29(24)11-12-31-8)27(6,7)25(30)28-26(3,4)5/h9-10,13-17H,11-12H2,1-8H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333710
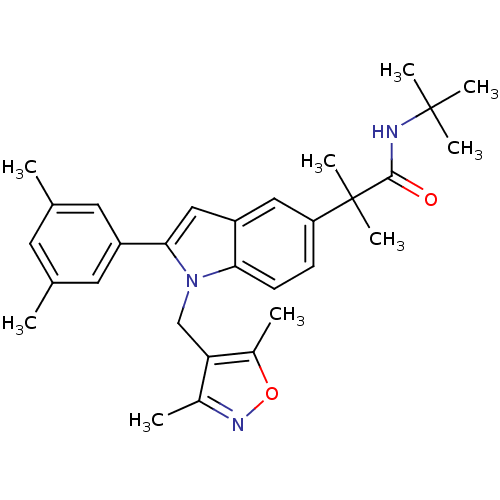
(CHEMBL1643726 | N-tert-butyl-2-(1-((3,5-dimethylis...)Show SMILES Cc1noc(C)c1Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C30H37N3O2/c1-18-12-19(2)14-22(13-18)27-16-23-15-24(30(8,9)28(34)31-29(5,6)7)10-11-26(23)33(27)17-25-20(3)32-35-21(25)4/h10-16H,17H2,1-9H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333712

((S)-tert-butyl 2-(3,5-dimethylphenyl)-5-(1-ethoxy-...)Show SMILES CCOC(=O)C(C)(C)c1ccc2n(C(=O)OC(C)(C)C)c(c([C@H](C)CO)c2c1)-c1cc(C)cc(C)c1 |r| Show InChI InChI=1S/C30H39NO5/c1-10-35-27(33)30(8,9)22-11-12-24-23(16-22)25(20(4)17-32)26(21-14-18(2)13-19(3)15-21)31(24)28(34)36-29(5,6)7/h11-16,20,32H,10,17H2,1-9H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333714
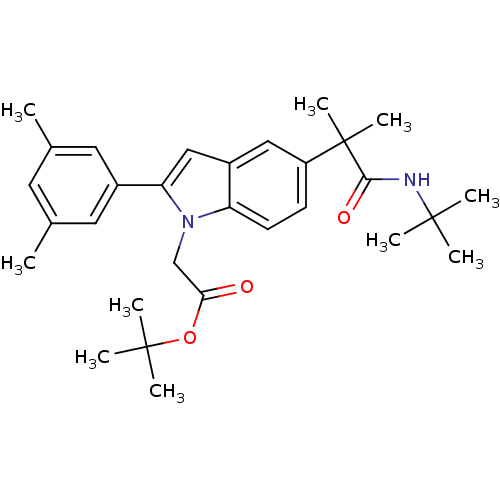
(CHEMBL1643723 | tert-butyl 2-(5-(1-(tert-butylamin...)Show SMILES Cc1cc(C)cc(c1)-c1cc2cc(ccc2n1CC(=O)OC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H40N2O3/c1-19-13-20(2)15-21(14-19)25-17-22-16-23(30(9,10)27(34)31-28(3,4)5)11-12-24(22)32(25)18-26(33)35-29(6,7)8/h11-17H,18H2,1-10H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333715

(CHEMBL1643720 | N-tert-butyl-2-(3,5-dimethylphenyl...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(=O)NC(C)(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C24H30N2O2/c1-15-9-16(2)11-19(10-15)22-13-20-12-18(23(28)25-24(4,5)6)7-8-21(20)26(22)14-17(3)27/h7-13,17,27H,14H2,1-6H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333717
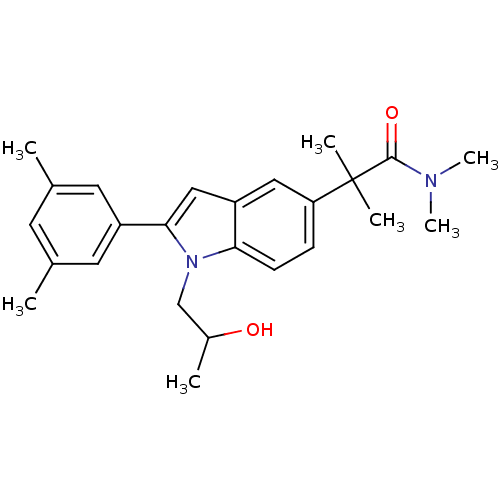
(2-(2-(3,5-dimethylphenyl)-1-(2-hydroxypropyl)-1H-i...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)N(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C25H32N2O2/c1-16-10-17(2)12-19(11-16)23-14-20-13-21(25(4,5)24(29)26(6)7)8-9-22(20)27(23)15-18(3)28/h8-14,18,28H,15H2,1-7H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333718

((S)-1-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(2-(3,5-d...)Show SMILES CCCN[C@@H](C)c1c([nH]c2ccc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1 |r,THB:18:20:22.23:25.26| Show InChI InChI=1S/C31H41N3O/c1-7-14-32-21(4)28-26-18-23(31(5,6)30(35)34-24-9-10-25(34)12-11-24)8-13-27(26)33-29(28)22-16-19(2)15-20(3)17-22/h8,13,15-18,21,24-25,32-33H,7,9-12,14H2,1-6H3/t21-,24?,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333720
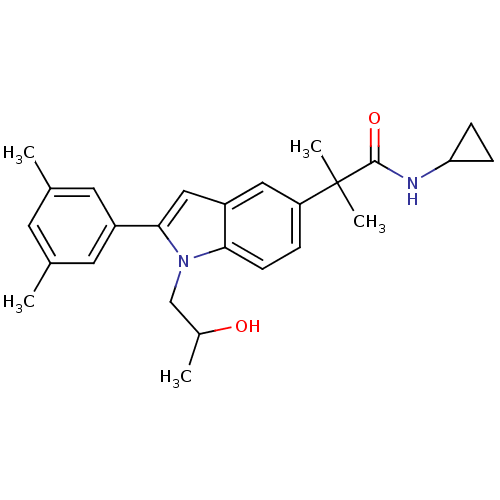
(CHEMBL1643718 | N-cyclopropyl-2-(2-(3,5-dimethylph...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC1CC1)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C26H32N2O2/c1-16-10-17(2)12-19(11-16)24-14-20-13-21(6-9-23(20)28(24)15-18(3)29)26(4,5)25(30)27-22-7-8-22/h6,9-14,18,22,29H,7-8,15H2,1-5H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333721

(2-(2-(3,5-dimethylphenyl)-1-(2-hydroxypropyl)-1H-i...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NCC(F)(F)F)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C25H29F3N2O2/c1-15-8-16(2)10-18(9-15)22-12-19-11-20(6-7-21(19)30(22)13-17(3)31)24(4,5)23(32)29-14-25(26,27)28/h6-12,17,31H,13-14H2,1-5H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50333708
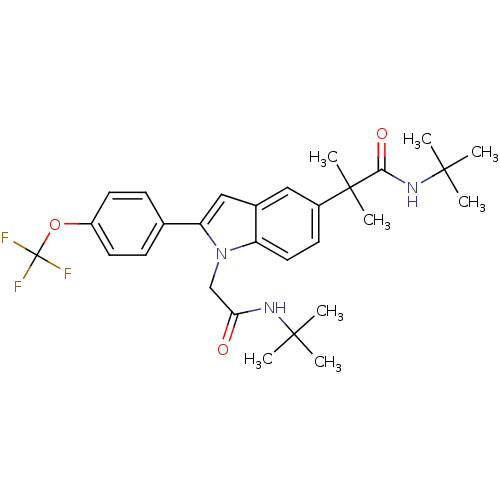
(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50333708

(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50333708

(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333723
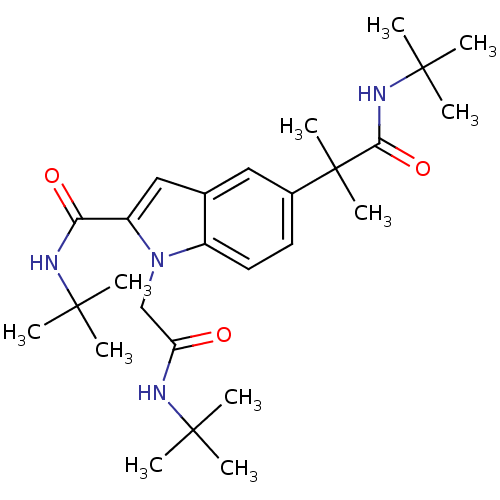
(CHEMBL1643738 | N-tert-butyl-5-(1-(tert-butylamino...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)C(=O)NC(C)(C)C Show InChI InChI=1S/C27H42N4O3/c1-24(2,3)28-21(32)16-31-19-13-12-18(27(10,11)23(34)30-26(7,8)9)14-17(19)15-20(31)22(33)29-25(4,5)6/h12-15H,16H2,1-11H3,(H,28,32)(H,29,33)(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.64E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333706

(CHEMBL1643733 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-21-14-13-19(28(7,8)25(37)34-27(4,5)6)15-18(21)16-22(35)20-11-9-10-12-23(20)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333708

(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333703

(CHEMBL1643724 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES Cc1cc(C)cc(c1)-c1cc2cc(ccc2n1CC(=O)NC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H41N3O2/c1-19-13-20(2)15-21(14-19)25-17-22-16-23(30(9,10)27(35)32-29(6,7)8)11-12-24(22)33(25)18-26(34)31-28(3,4)5/h11-17H,18H2,1-10H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333705
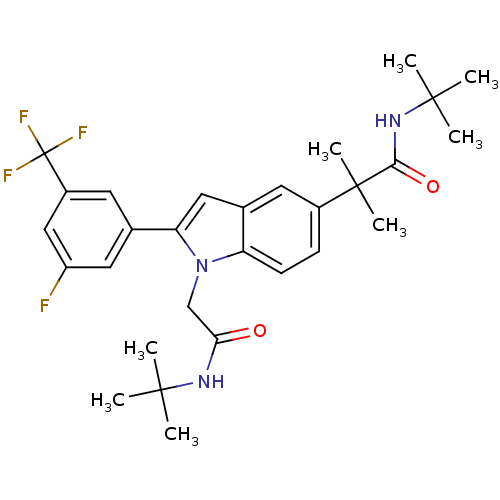
(CHEMBL1643731 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(F)cc(c1)C(F)(F)F Show InChI InChI=1S/C29H35F4N3O2/c1-26(2,3)34-24(37)16-36-22-10-9-19(28(7,8)25(38)35-27(4,5)6)11-18(22)14-23(36)17-12-20(29(31,32)33)15-21(30)13-17/h9-15H,16H2,1-8H3,(H,34,37)(H,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333719

(CHEMBL1643732 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(F)cc1F Show InChI InChI=1S/C28H35F2N3O2/c1-26(2,3)31-24(34)16-33-22-12-9-18(28(7,8)25(35)32-27(4,5)6)13-17(22)14-23(33)20-11-10-19(29)15-21(20)30/h9-15H,16H2,1-8H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 940 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333722

(CHEMBL1643728 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccccc1 Show InChI InChI=1S/C28H37N3O2/c1-26(2,3)29-24(32)18-31-22-15-14-21(28(7,8)25(33)30-27(4,5)6)16-20(22)17-23(31)19-12-10-9-11-13-19/h9-17H,18H2,1-8H3,(H,29,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333707

(CHEMBL1643734 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-13-12-20(28(7,8)25(37)34-27(4,5)6)14-19(22)16-23(35)18-10-9-11-21(15-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333713
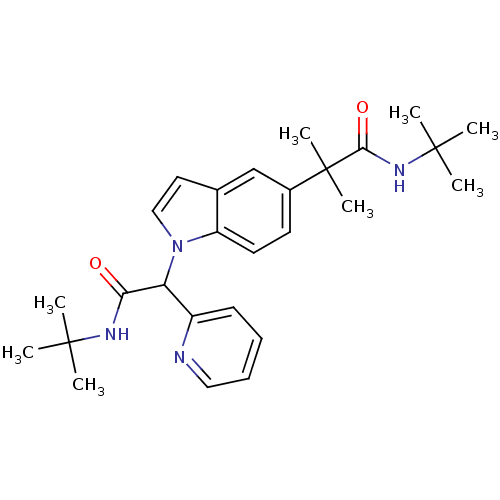
(CHEMBL1643739 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)C(c1ccccn1)n1ccc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N4O2/c1-25(2,3)29-23(32)22(20-11-9-10-15-28-20)31-16-14-18-17-19(12-13-21(18)31)27(7,8)24(33)30-26(4,5)6/h9-17,22H,1-8H3,(H,29,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333724
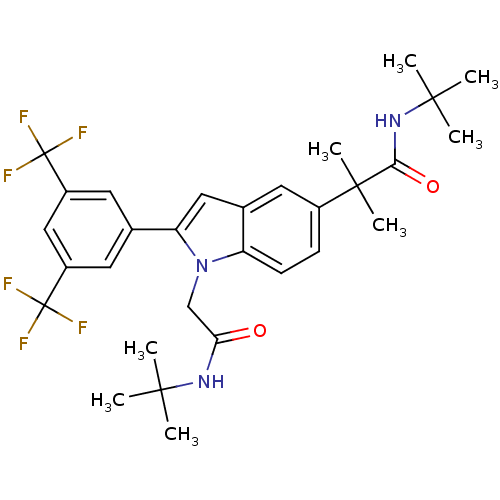
(2-(2-(3,5-bis(trifluoromethyl)phenyl)-1-(2-(tert-b...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C30H35F6N3O2/c1-26(2,3)37-24(40)16-39-22-10-9-19(28(7,8)25(41)38-27(4,5)6)11-18(22)14-23(39)17-12-20(29(31,32)33)15-21(13-17)30(34,35)36/h9-15H,16H2,1-8H3,(H,37,40)(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333704
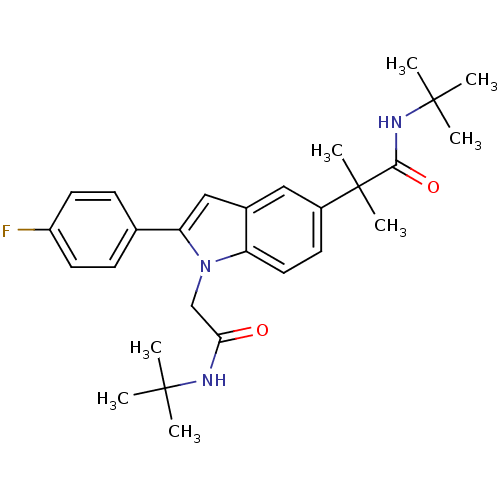
(CHEMBL1643729 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(F)cc1 Show InChI InChI=1S/C28H36FN3O2/c1-26(2,3)30-24(33)17-32-22-14-11-20(28(7,8)25(34)31-27(4,5)6)15-19(22)16-23(32)18-9-12-21(29)13-10-18/h9-16H,17H2,1-8H3,(H,30,33)(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333711

(CHEMBL1643737 | N-tert-butyl-2-(2-tert-butyl-1-(2-...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H41N3O2/c1-23(2,3)20-15-17-14-18(26(10,11)22(31)28-25(7,8)9)12-13-19(17)29(20)16-21(30)27-24(4,5)6/h12-15H,16H2,1-11H3,(H,27,30)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333716
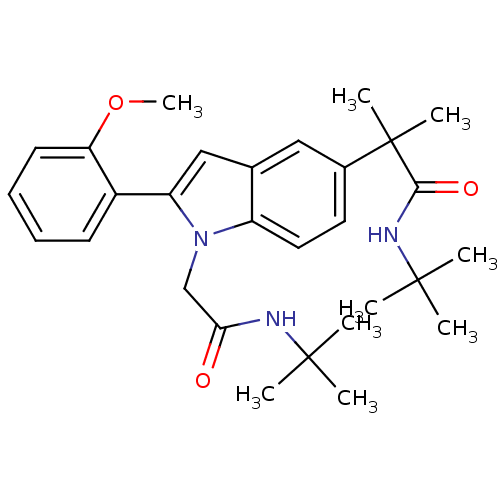
(CHEMBL1643736 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES COc1ccccc1-c1cc2cc(ccc2n1CC(=O)NC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C29H39N3O3/c1-27(2,3)30-25(33)18-32-22-15-14-20(29(7,8)26(34)31-28(4,5)6)16-19(22)17-23(32)21-12-10-11-13-24(21)35-9/h10-17H,18H2,1-9H3,(H,30,33)(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data