 Found 44 hits of Enzyme Inhibition Constant Data
Found 44 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336345
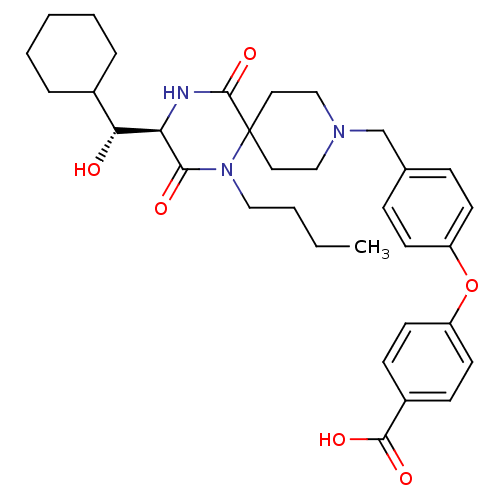
(4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50198936
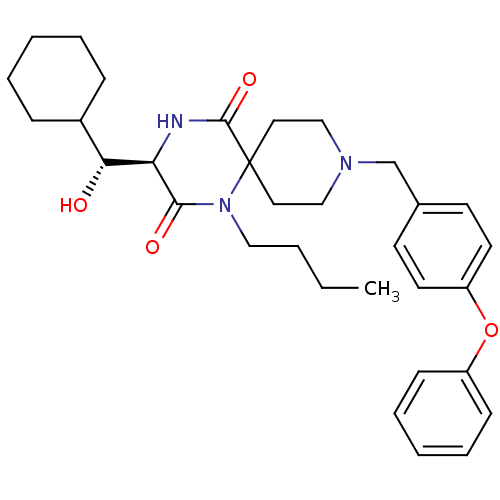
((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C1CCCCC1 Show InChI InChI=1S/C32H43N3O4/c1-2-3-20-35-30(37)28(29(36)25-10-6-4-7-11-25)33-31(38)32(35)18-21-34(22-19-32)23-24-14-16-27(17-15-24)39-26-12-8-5-9-13-26/h5,8-9,12-17,25,28-29,36H,2-4,6-7,10-11,18-23H2,1H3,(H,33,38)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50322288
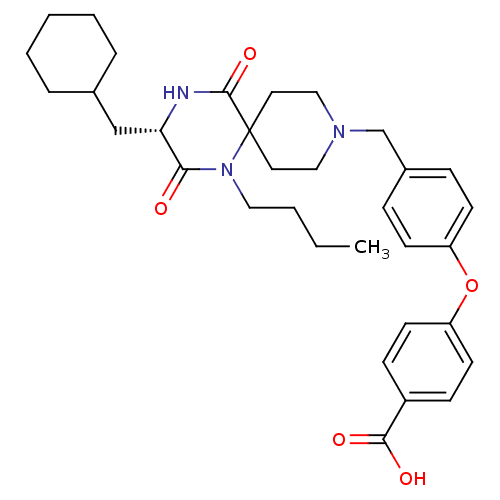
(4-(4-{[(3S)-1-Butyl-3-(cyclohexylmethyl)-2,5-dioxo...)Show SMILES CCCCN1C(=O)[C@H](CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1 |r| Show InChI InChI=1S/C33H43N3O5/c1-2-3-19-36-30(37)29(22-24-7-5-4-6-8-24)34-32(40)33(36)17-20-35(21-18-33)23-25-9-13-27(14-10-25)41-28-15-11-26(12-16-28)31(38)39/h9-16,24,29H,2-8,17-23H2,1H3,(H,34,40)(H,38,39)/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50190516
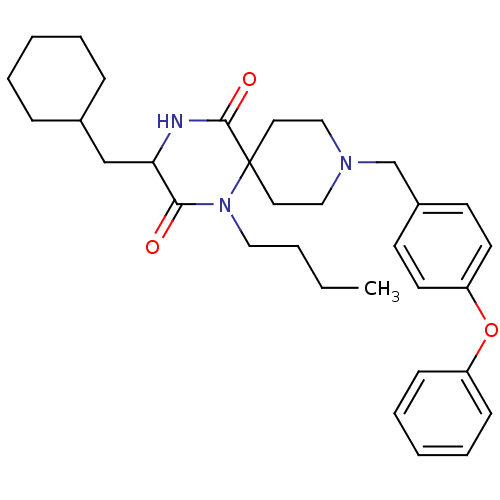
((RS)-1-butyl-3-(cyclohexylmethyl)-9-(4-phenoxybenz...)Show SMILES CCCCN1C(=O)C(CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C32H43N3O3/c1-2-3-20-35-30(36)29(23-25-10-6-4-7-11-25)33-31(37)32(35)18-21-34(22-19-32)24-26-14-16-28(17-15-26)38-27-12-8-5-9-13-27/h5,8-9,12-17,25,29H,2-4,6-7,10-11,18-24H2,1H3,(H,33,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336345

(4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336346
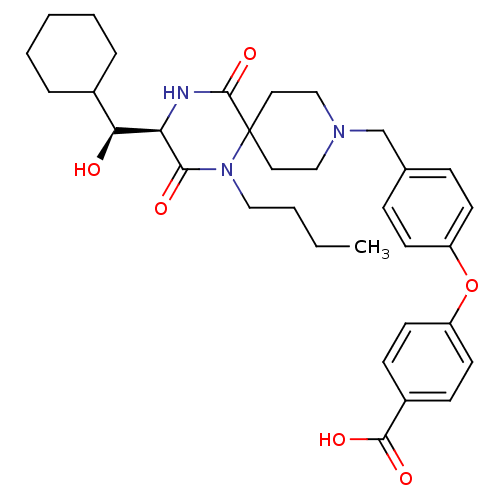
(4-(4-(((R)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336348
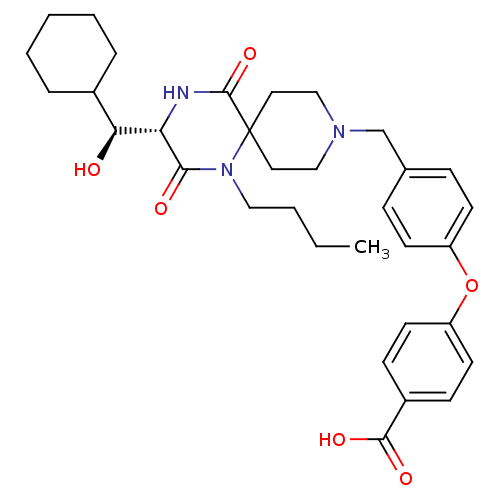
(4-(4-(((S)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50198936

((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C1CCCCC1 Show InChI InChI=1S/C32H43N3O4/c1-2-3-20-35-30(37)28(29(36)25-10-6-4-7-11-25)33-31(38)32(35)18-21-34(22-19-32)23-24-14-16-27(17-15-24)39-26-12-8-5-9-13-26/h5,8-9,12-17,25,28-29,36H,2-4,6-7,10-11,18-23H2,1H3,(H,33,38)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50198939
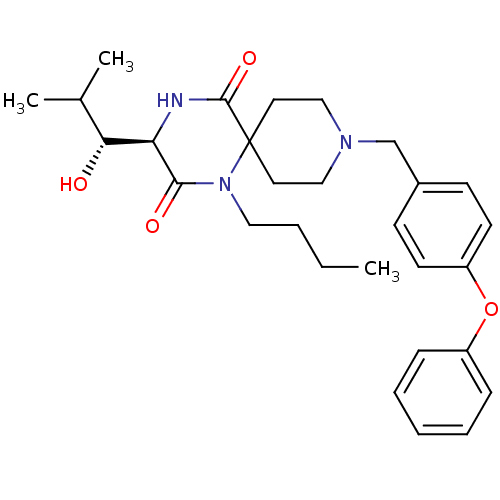
((R)-1-butyl-3-((R)-1-hydroxy-2-methyl-propyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C(C)C Show InChI InChI=1S/C29H39N3O4/c1-4-5-17-32-27(34)25(26(33)21(2)3)30-28(35)29(32)15-18-31(19-16-29)20-22-11-13-24(14-12-22)36-23-9-7-6-8-10-23/h6-14,21,25-26,33H,4-5,15-20H2,1-3H3,(H,30,35)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50322288

(4-(4-{[(3S)-1-Butyl-3-(cyclohexylmethyl)-2,5-dioxo...)Show SMILES CCCCN1C(=O)[C@H](CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1 |r| Show InChI InChI=1S/C33H43N3O5/c1-2-3-19-36-30(37)29(22-24-7-5-4-6-8-24)34-32(40)33(36)17-20-35(21-18-33)23-25-9-13-27(14-10-25)41-28-15-11-26(12-16-28)31(38)39/h9-16,24,29H,2-8,17-23H2,1H3,(H,34,40)(H,38,39)/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50190520
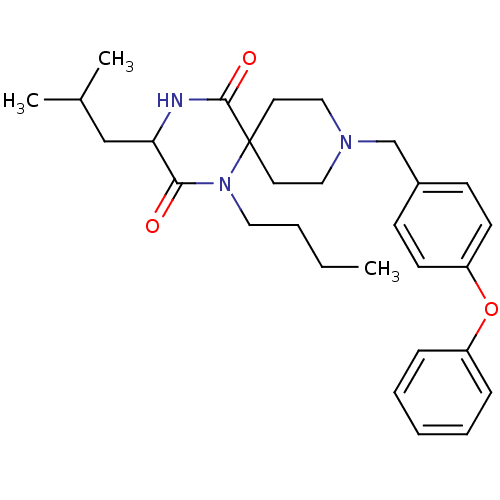
((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...)Show SMILES CCCCN1C(=O)C(CC(C)C)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C29H39N3O3/c1-4-5-17-32-27(33)26(20-22(2)3)30-28(34)29(32)15-18-31(19-16-29)21-23-11-13-25(14-12-23)35-24-9-7-6-8-10-24/h6-14,22,26H,4-5,15-21H2,1-3H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336346

(4-(4-(((R)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50198939

((R)-1-butyl-3-((R)-1-hydroxy-2-methyl-propyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C(C)C Show InChI InChI=1S/C29H39N3O4/c1-4-5-17-32-27(34)25(26(33)21(2)3)30-28(35)29(32)15-18-31(19-16-29)20-22-11-13-24(14-12-22)36-23-9-7-6-8-10-23/h6-14,21,25-26,33H,4-5,15-20H2,1-3H3,(H,30,35)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336347
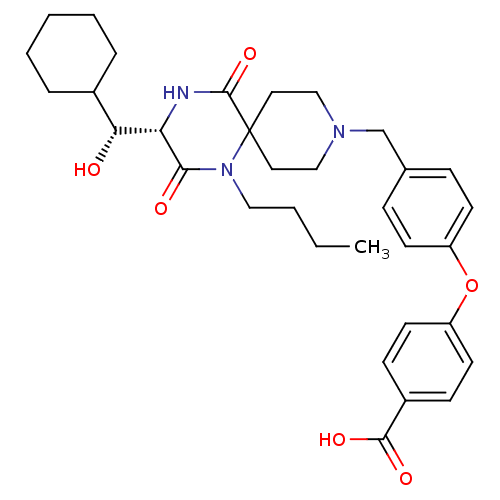
(4-(4-(((S)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336348

(4-(4-(((S)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50336347

(4-(4-(((S)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization Ca assay |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50190520

((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...)Show SMILES CCCCN1C(=O)C(CC(C)C)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C29H39N3O3/c1-4-5-17-32-27(33)26(20-22(2)3)30-28(34)29(32)15-18-31(19-16-29)21-23-11-13-25(14-12-23)35-24-9-7-6-8-10-24/h6-14,22,26H,4-5,15-21H2,1-3H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 expressed in HOS cells assessed as inhibition of cell fusion with HIV gp120 expressing HEK293 cells by LTR lucifera... |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50198936

((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C1CCCCC1 Show InChI InChI=1S/C32H43N3O4/c1-2-3-20-35-30(37)28(29(36)25-10-6-4-7-11-25)33-31(38)32(35)18-21-34(22-19-32)23-24-14-16-27(17-15-24)39-26-12-8-5-9-13-26/h5,8-9,12-17,25,28-29,36H,2-4,6-7,10-11,18-23H2,1H3,(H,33,38)/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50190520

((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...)Show SMILES CCCCN1C(=O)C(CC(C)C)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C29H39N3O3/c1-4-5-17-32-27(33)26(20-22(2)3)30-28(34)29(32)15-18-31(19-16-29)21-23-11-13-25(14-12-23)35-24-9-7-6-8-10-24/h6-14,22,26H,4-5,15-21H2,1-3H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50190516

((RS)-1-butyl-3-(cyclohexylmethyl)-9-(4-phenoxybenz...)Show SMILES CCCCN1C(=O)C(CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C32H43N3O3/c1-2-3-20-35-30(36)29(23-25-10-6-4-7-11-25)33-31(37)32(35)18-21-34(22-19-32)24-26-14-16-28(17-15-26)38-27-12-8-5-9-13-27/h5,8-9,12-17,25,29H,2-4,6-7,10-11,18-24H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50190520

((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...)Show SMILES CCCCN1C(=O)C(CC(C)C)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C29H39N3O3/c1-4-5-17-32-27(33)26(20-22(2)3)30-28(34)29(32)15-18-31(19-16-29)21-23-11-13-25(14-12-23)35-24-9-7-6-8-10-24/h6-14,22,26H,4-5,15-21H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50190516

((RS)-1-butyl-3-(cyclohexylmethyl)-9-(4-phenoxybenz...)Show SMILES CCCCN1C(=O)C(CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C32H43N3O3/c1-2-3-20-35-30(36)29(23-25-10-6-4-7-11-25)33-31(37)32(35)18-21-34(22-19-32)24-26-14-16-28(17-15-26)38-27-12-8-5-9-13-27/h5,8-9,12-17,25,29H,2-4,6-7,10-11,18-24H2,1H3,(H,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50322288

(4-(4-{[(3S)-1-Butyl-3-(cyclohexylmethyl)-2,5-dioxo...)Show SMILES CCCCN1C(=O)[C@H](CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1 |r| Show InChI InChI=1S/C33H43N3O5/c1-2-3-19-36-30(37)29(22-24-7-5-4-6-8-24)34-32(40)33(36)17-20-35(21-18-33)23-25-9-13-27(14-10-25)41-28-15-11-26(12-16-28)31(38)39/h9-16,24,29H,2-8,17-23H2,1H3,(H,34,40)(H,38,39)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50190516

((RS)-1-butyl-3-(cyclohexylmethyl)-9-(4-phenoxybenz...)Show SMILES CCCCN1C(=O)C(CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C32H43N3O3/c1-2-3-20-35-30(36)29(23-25-10-6-4-7-11-25)33-31(37)32(35)18-21-34(22-19-32)24-26-14-16-28(17-15-26)38-27-12-8-5-9-13-27/h5,8-9,12-17,25,29H,2-4,6-7,10-11,18-24H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50198936

((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C1CCCCC1 Show InChI InChI=1S/C32H43N3O4/c1-2-3-20-35-30(37)28(29(36)25-10-6-4-7-11-25)33-31(38)32(35)18-21-34(22-19-32)23-24-14-16-27(17-15-24)39-26-12-8-5-9-13-26/h5,8-9,12-17,25,28-29,36H,2-4,6-7,10-11,18-23H2,1H3,(H,33,38)/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50336346

(4-(4-(((R)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50190520

((RS)-1-butyl-3-isobutyl-9-(4-phenoxybenzyl)-1,4,9-...)Show SMILES CCCCN1C(=O)C(CC(C)C)NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1 Show InChI InChI=1S/C29H39N3O3/c1-4-5-17-32-27(33)26(20-22(2)3)30-28(34)29(32)15-18-31(19-16-29)21-23-11-13-25(14-12-23)35-24-9-7-6-8-10-24/h6-14,22,26H,4-5,15-21H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50322288

(4-(4-{[(3S)-1-Butyl-3-(cyclohexylmethyl)-2,5-dioxo...)Show SMILES CCCCN1C(=O)[C@H](CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1 |r| Show InChI InChI=1S/C33H43N3O5/c1-2-3-19-36-30(37)29(22-24-7-5-4-6-8-24)34-32(40)33(36)17-20-35(21-18-33)23-25-9-13-27(14-10-25)41-28-15-11-26(12-16-28)31(38)39/h9-16,24,29H,2-8,17-23H2,1H3,(H,34,40)(H,38,39)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336348

(4-(4-(((S)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336347

(4-(4-(((S)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336348

(4-(4-(((S)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50322288

(4-(4-{[(3S)-1-Butyl-3-(cyclohexylmethyl)-2,5-dioxo...)Show SMILES CCCCN1C(=O)[C@H](CC2CCCCC2)NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1 |r| Show InChI InChI=1S/C33H43N3O5/c1-2-3-19-36-30(37)29(22-24-7-5-4-6-8-24)34-32(40)33(36)17-20-35(21-18-33)23-25-9-13-27(14-10-25)41-28-15-11-26(12-16-28)31(38)39/h9-16,24,29H,2-8,17-23H2,1H3,(H,34,40)(H,38,39)/t29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336346

(4-(4-(((R)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50198936

((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C1CCCCC1 Show InChI InChI=1S/C32H43N3O4/c1-2-3-20-35-30(37)28(29(36)25-10-6-4-7-11-25)33-31(38)32(35)18-21-34(22-19-32)23-24-14-16-27(17-15-24)39-26-12-8-5-9-13-26/h5,8-9,12-17,25,28-29,36H,2-4,6-7,10-11,18-23H2,1H3,(H,33,38)/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50198939

((R)-1-butyl-3-((R)-1-hydroxy-2-methyl-propyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C(C)C Show InChI InChI=1S/C29H39N3O4/c1-4-5-17-32-27(34)25(26(33)21(2)3)30-28(35)29(32)15-18-31(19-16-29)20-22-11-13-24(14-12-22)36-23-9-7-6-8-10-23/h6-14,21,25-26,33H,4-5,15-20H2,1-3H3,(H,30,35)/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336347

(4-(4-(((S)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336345

(4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336346

(4-(4-(((R)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50198939

((R)-1-butyl-3-((R)-1-hydroxy-2-methyl-propyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C(C)C Show InChI InChI=1S/C29H39N3O4/c1-4-5-17-32-27(34)25(26(33)21(2)3)30-28(35)29(32)15-18-31(19-16-29)20-22-11-13-24(14-12-22)36-23-9-7-6-8-10-23/h6-14,21,25-26,33H,4-5,15-20H2,1-3H3,(H,30,35)/t25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50336348

(4-(4-(((S)-1-butyl-3-((S)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50198939

((R)-1-butyl-3-((R)-1-hydroxy-2-methyl-propyl)-9-(4...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccccc3)cc2)CC1)[C@H](O)C(C)C Show InChI InChI=1S/C29H39N3O4/c1-4-5-17-32-27(34)25(26(33)21(2)3)30-28(35)29(32)15-18-31(19-16-29)20-22-11-13-24(14-12-22)36-23-9-7-6-8-10-23/h6-14,21,25-26,33H,4-5,15-20H2,1-3H3,(H,30,35)/t25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336345

(4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50336345

(4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50336347

(4-(4-(((S)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...)Show SMILES CCCCN1C(=O)[C@@H](NC(=O)C11CCN(Cc2ccc(Oc3ccc(cc3)C(O)=O)cc2)CC1)[C@H](O)C1CCCCC1 |r| Show InChI InChI=1S/C33H43N3O6/c1-2-3-19-36-30(38)28(29(37)24-7-5-4-6-8-24)34-32(41)33(36)17-20-35(21-18-33)22-23-9-13-26(14-10-23)42-27-15-11-25(12-16-27)31(39)40/h9-16,24,28-29,37H,2-8,17-22H2,1H3,(H,34,41)(H,39,40)/t28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1141-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.109
BindingDB Entry DOI: 10.7270/Q24T6JP0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data