 Found 71 hits of Enzyme Inhibition Constant Data
Found 71 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337251
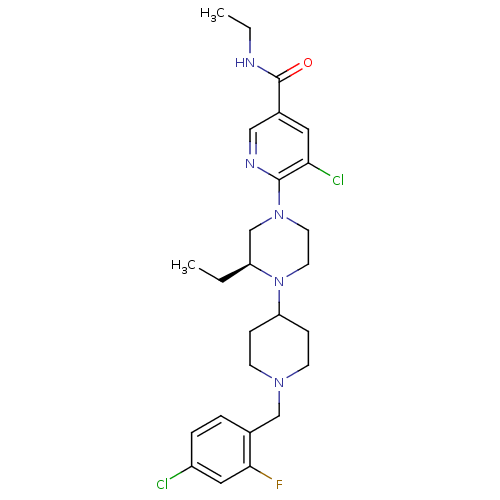
((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250
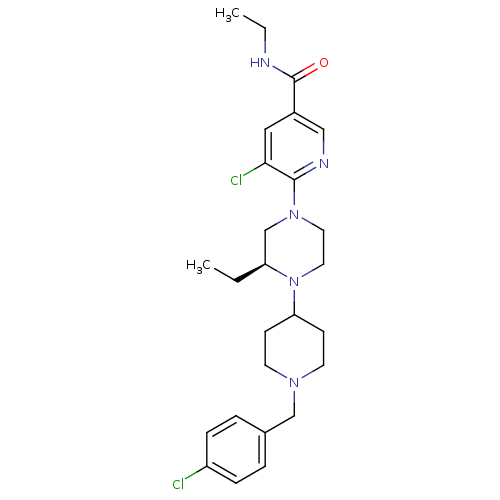
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337251

((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337251

((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337236
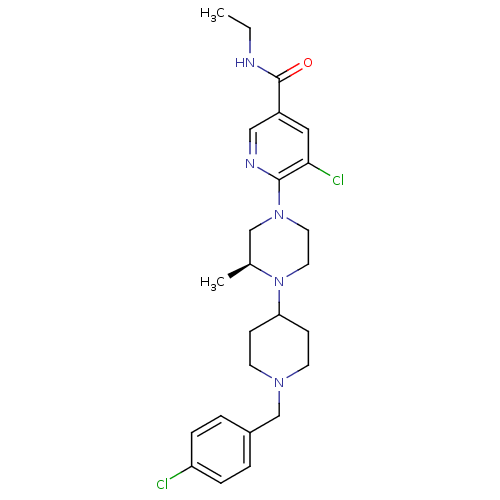
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-3-28-25(33)20-14-23(27)24(29-15-20)31-12-13-32(18(2)16-31)22-8-10-30(11-9-22)17-19-4-6-21(26)7-5-19/h4-7,14-15,18,22H,3,8-13,16-17H2,1-2H3,(H,28,33)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337238
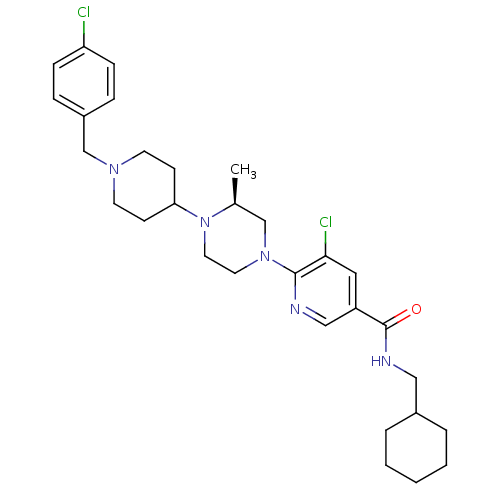
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCC1CCCCC1 |r| Show InChI InChI=1S/C30H41Cl2N5O/c1-22-20-36(29-28(32)17-25(19-33-29)30(38)34-18-23-5-3-2-4-6-23)15-16-37(22)27-11-13-35(14-12-27)21-24-7-9-26(31)10-8-24/h7-10,17,19,22-23,27H,2-6,11-16,18,20-21H2,1H3,(H,34,38)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337242
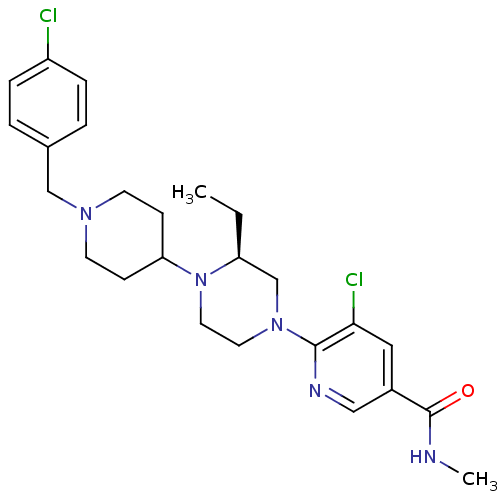
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NC |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-3-21-17-31(24-23(27)14-19(15-29-24)25(33)28-2)12-13-32(21)22-8-10-30(11-9-22)16-18-4-6-20(26)7-5-18/h4-7,14-15,21-22H,3,8-13,16-17H2,1-2H3,(H,28,33)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337237

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CC(C)NC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-18(2)30-26(34)21-14-24(28)25(29-15-21)32-12-13-33(19(3)16-32)23-8-10-31(11-9-23)17-20-4-6-22(27)7-5-20/h4-7,14-15,18-19,23H,8-13,16-17H2,1-3H3,(H,30,34)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337212
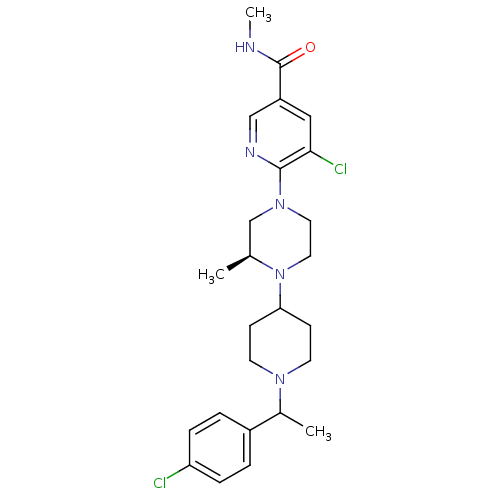
(5-chloro-6-((3S)-4-(1-(1-(4-chlorophenyl)ethyl)pip...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(C)c2ccc(Cl)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-17-16-31(24-23(27)14-20(15-29-24)25(33)28-3)12-13-32(17)22-8-10-30(11-9-22)18(2)19-4-6-21(26)7-5-19/h4-7,14-15,17-18,22H,8-13,16H2,1-3H3,(H,28,33)/t17-,18?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337212

(5-chloro-6-((3S)-4-(1-(1-(4-chlorophenyl)ethyl)pip...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(C)c2ccc(Cl)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-17-16-31(24-23(27)14-20(15-29-24)25(33)28-3)12-13-32(17)22-8-10-30(11-9-22)18(2)19-4-6-21(26)7-5-19/h4-7,14-15,17-18,22H,8-13,16H2,1-3H3,(H,28,33)/t17-,18?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337233
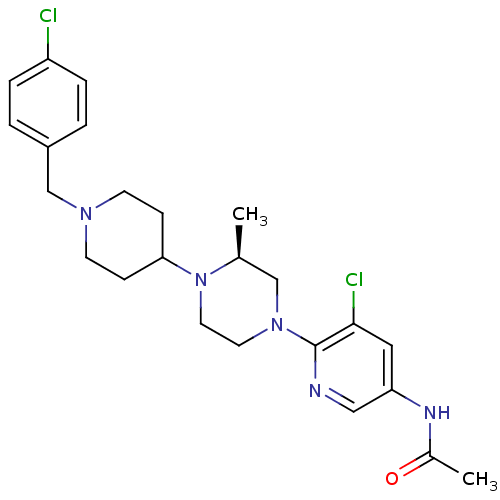
((S)-N-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(NC(C)=O)cc1Cl |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-30(24-23(26)13-21(14-27-24)28-18(2)32)11-12-31(17)22-7-9-29(10-8-22)16-19-3-5-20(25)6-4-19/h3-6,13-14,17,22H,7-12,15-16H2,1-2H3,(H,28,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337219
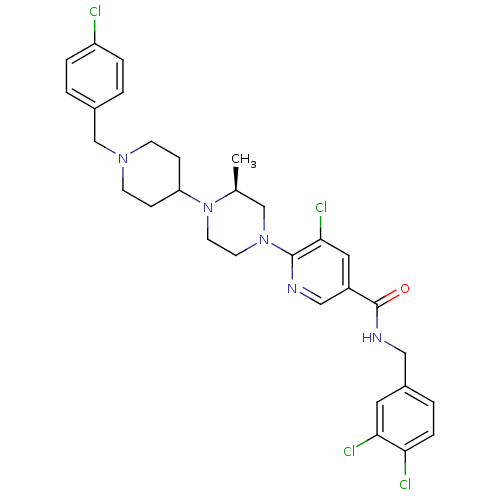
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H33Cl4N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-28(34)15-23(17-35-29)30(40)36-16-22-4-7-26(32)27(33)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337210
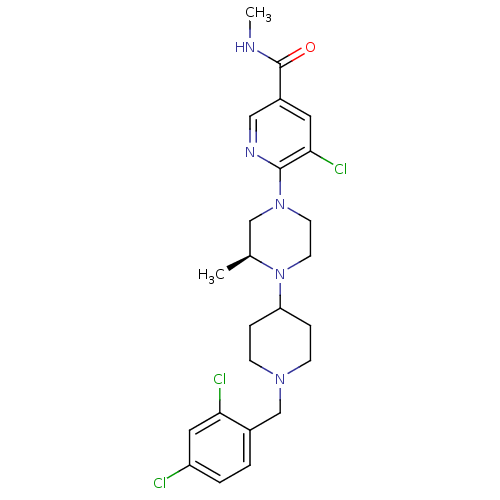
((S)-5-chloro-6-(4-(1-(2,4-dichlorobenzyl)piperidin...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3Cl)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C24H30Cl3N5O/c1-16-14-31(23-22(27)11-18(13-29-23)24(33)28-2)9-10-32(16)20-5-7-30(8-6-20)15-17-3-4-19(25)12-21(17)26/h3-4,11-13,16,20H,5-10,14-15H2,1-2H3,(H,28,33)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337262
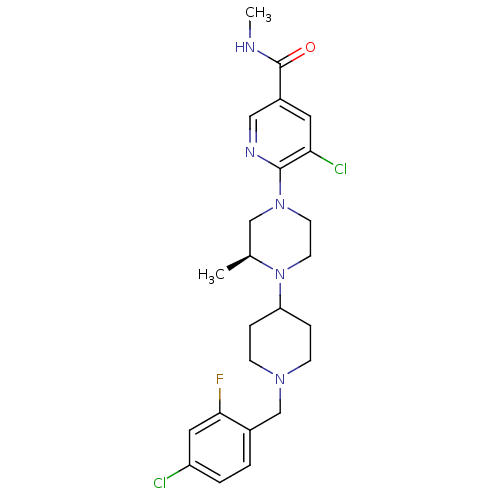
((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C24H30Cl2FN5O/c1-16-14-31(23-21(26)11-18(13-29-23)24(33)28-2)9-10-32(16)20-5-7-30(8-6-20)15-17-3-4-19(25)12-22(17)27/h3-4,11-13,16,20H,5-10,14-15H2,1-2H3,(H,28,33)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337234
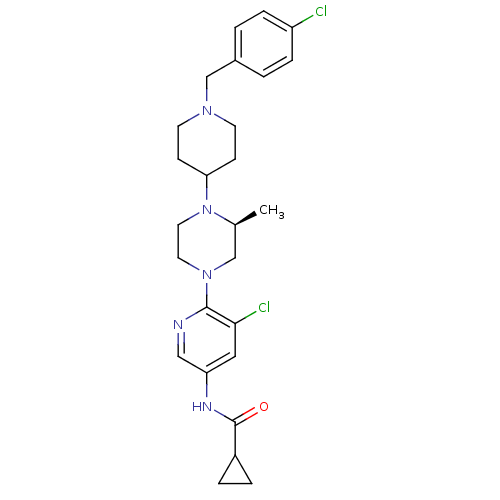
((S)-N-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(NC(=O)C2CC2)cc1Cl |r| Show InChI InChI=1S/C26H33Cl2N5O/c1-18-16-32(25-24(28)14-22(15-29-25)30-26(34)20-4-5-20)12-13-33(18)23-8-10-31(11-9-23)17-19-2-6-21(27)7-3-19/h2-3,6-7,14-15,18,20,23H,4-5,8-13,16-17H2,1H3,(H,30,34)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337239

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)Nc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C29H31Cl2F2N5O/c1-19-17-37(12-13-38(19)24-8-10-36(11-9-24)18-20-2-4-22(30)5-3-20)28-25(31)14-21(16-34-28)29(39)35-23-6-7-26(32)27(33)15-23/h2-7,14-16,19,24H,8-13,17-18H2,1H3,(H,35,39)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337252
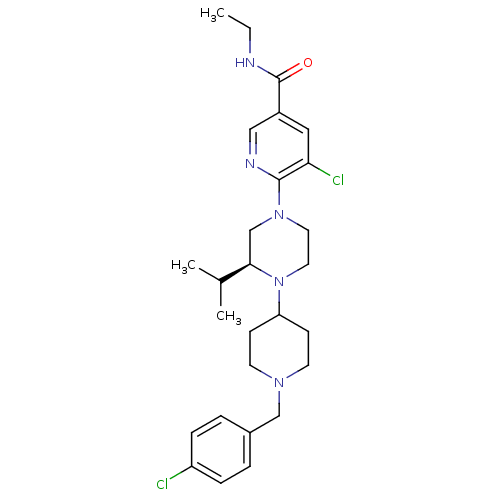
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN(C3CCN(Cc4ccc(Cl)cc4)CC3)[C@H](C2)C(C)C)c(Cl)c1 |r| Show InChI InChI=1S/C27H37Cl2N5O/c1-4-30-27(35)21-15-24(29)26(31-16-21)33-13-14-34(25(18-33)19(2)3)23-9-11-32(12-10-23)17-20-5-7-22(28)8-6-20/h5-8,15-16,19,23,25H,4,9-14,17-18H2,1-3H3,(H,30,35)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CXCR3 by chemotaxis assay |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337224

(5-chloro-6-(4-(1-(4-chlorobenzyl)-4-methylpiperidi...)Show SMILES CC1(CCN(Cc2ccc(Cl)cc2)CC1)N1CCN(CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H33Cl4N5O/c1-30(8-10-37(11-9-30)20-21-2-5-24(31)6-3-21)39-14-12-38(13-15-39)28-27(34)17-23(19-35-28)29(40)36-18-22-4-7-25(32)26(33)16-22/h2-7,16-17,19H,8-15,18,20H2,1H3,(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337213
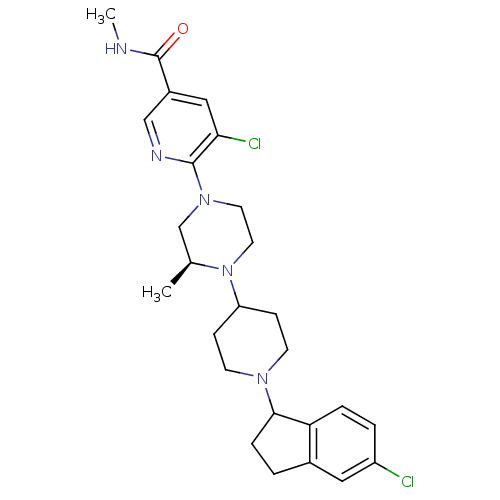
(5-chloro-6-((3S)-4-(1-(5-chloro-2,3-dihydro-1H-ind...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C2CCc3cc(Cl)ccc23)c(Cl)c1 |r| Show InChI InChI=1S/C26H33Cl2N5O/c1-17-16-32(25-23(28)14-19(15-30-25)26(34)29-2)11-12-33(17)21-7-9-31(10-8-21)24-6-3-18-13-20(27)4-5-22(18)24/h4-5,13-15,17,21,24H,3,6-12,16H2,1-2H3,(H,29,34)/t17-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337213

(5-chloro-6-((3S)-4-(1-(5-chloro-2,3-dihydro-1H-ind...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C2CCc3cc(Cl)ccc23)c(Cl)c1 |r| Show InChI InChI=1S/C26H33Cl2N5O/c1-17-16-32(25-23(28)14-19(15-30-25)26(34)29-2)11-12-33(17)21-7-9-31(10-8-21)24-6-3-18-13-20(27)4-5-22(18)24/h4-5,13-15,17,21,24H,3,6-12,16H2,1-2H3,(H,29,34)/t17-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301330
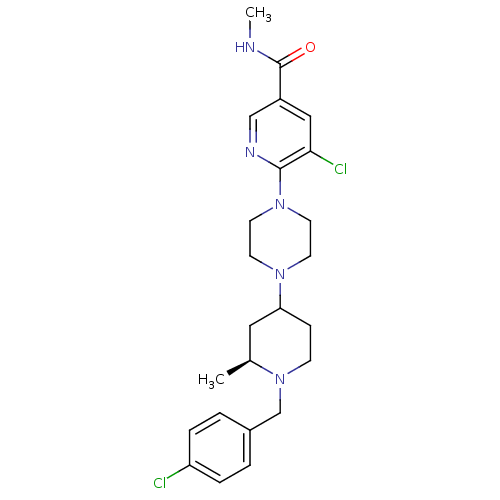
(5-chloro-6-(4-((2S)-1-(4-chlorobenzyl)-2-methylpip...)Show SMILES CNC(=O)c1cnc(N2CCN(CC2)C2CCN(Cc3ccc(Cl)cc3)[C@@H](C)C2)c(Cl)c1 |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-13-21(7-8-31(17)16-18-3-5-20(25)6-4-18)29-9-11-30(12-10-29)23-22(26)14-19(15-28-23)24(32)27-2/h3-6,14-15,17,21H,7-13,16H2,1-2H3,(H,27,32)/t17-,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337254
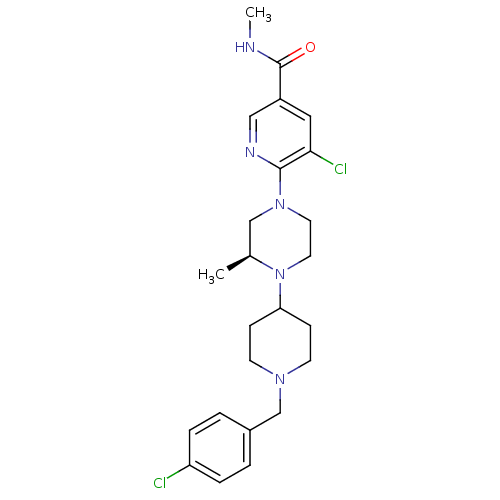
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-30(23-22(26)13-19(14-28-23)24(32)27-2)11-12-31(17)21-7-9-29(10-8-21)16-18-3-5-20(25)6-4-18/h3-6,13-14,17,21H,7-12,15-16H2,1-2H3,(H,27,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337253
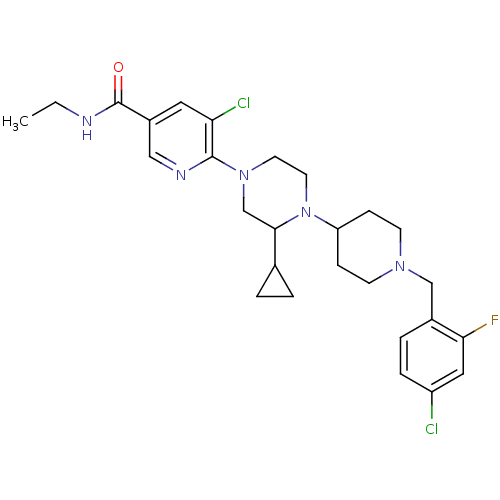
(5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)piperidi...)Show SMILES CCNC(=O)c1cnc(N2CCN(C3CCN(Cc4ccc(Cl)cc4F)CC3)C(C2)C2CC2)c(Cl)c1 Show InChI InChI=1S/C27H34Cl2FN5O/c1-2-31-27(36)20-13-23(29)26(32-15-20)34-11-12-35(25(17-34)18-3-4-18)22-7-9-33(10-8-22)16-19-5-6-21(28)14-24(19)30/h5-6,13-15,18,22,25H,2-4,7-12,16-17H2,1H3,(H,31,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301351
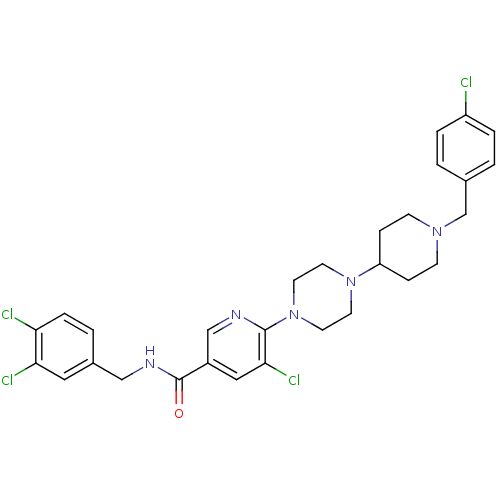
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES Clc1ccc(CN2CCC(CC2)N2CCN(CC2)c2ncc(cc2Cl)C(=O)NCc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C29H31Cl4N5O/c30-23-4-1-20(2-5-23)19-36-9-7-24(8-10-36)37-11-13-38(14-12-37)28-27(33)16-22(18-34-28)29(39)35-17-21-3-6-25(31)26(32)15-21/h1-6,15-16,18,24H,7-14,17,19H2,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337220
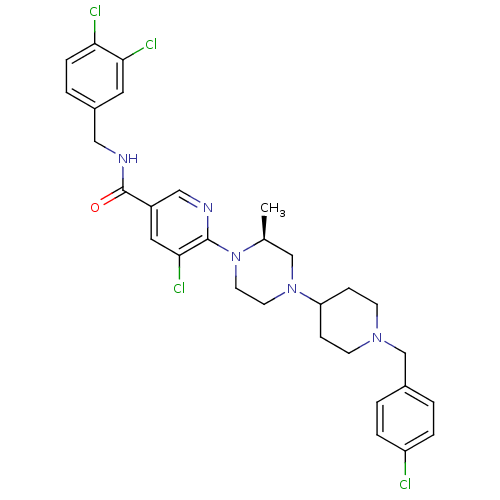
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1)C1CCN(Cc2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C30H33Cl4N5O/c1-20-18-38(25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)12-13-39(20)29-28(34)15-23(17-35-29)30(40)36-16-22-4-7-26(32)27(33)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337235
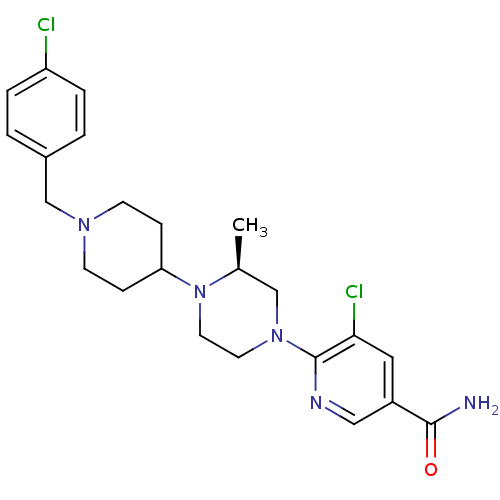
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(N)=O |r| Show InChI InChI=1S/C23H29Cl2N5O/c1-16-14-29(23-21(25)12-18(13-27-23)22(26)31)10-11-30(16)20-6-8-28(9-7-20)15-17-2-4-19(24)5-3-17/h2-5,12-13,16,20H,6-11,14-15H2,1H3,(H2,26,31)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337217

(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES Fc1ccc(CNC(=O)c2cnc(N3CCN(CC3)C3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)c2)cc1F Show InChI InChI=1S/C29H31Cl2F2N5O/c30-23-4-1-20(2-5-23)19-36-9-7-24(8-10-36)37-11-13-38(14-12-37)28-25(31)16-22(18-34-28)29(39)35-17-21-3-6-26(32)27(33)15-21/h1-6,15-16,18,24H,7-14,17,19H2,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337211

((S)-5-chloro-6-(4-(1-(2-chloro-4-fluorobenzyl)pipe...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(F)cc3Cl)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C24H30Cl2FN5O/c1-16-14-31(23-22(26)11-18(13-29-23)24(33)28-2)9-10-32(16)20-5-7-30(8-6-20)15-17-3-4-19(27)12-21(17)25/h3-4,11-13,16,20H,5-10,14-15H2,1-2H3,(H,28,33)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337261
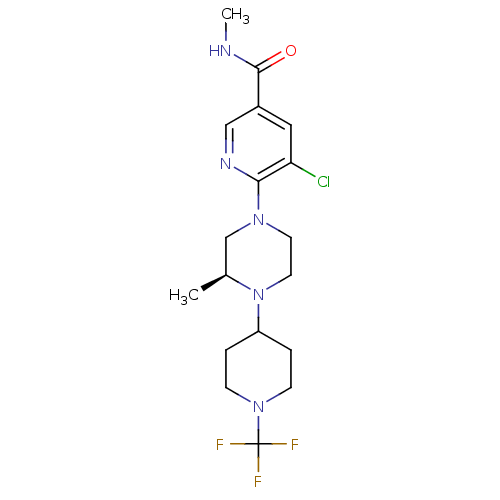
((S)-5-chloro-N-methyl-6-(3-methyl-4-(1-(trifluorom...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(F)(F)F)c(Cl)c1 |r| Show InChI InChI=1S/C18H25ClF3N5O/c1-12-11-25(16-15(19)9-13(10-24-16)17(28)23-2)7-8-27(12)14-3-5-26(6-4-14)18(20,21)22/h9-10,12,14H,3-8,11H2,1-2H3,(H,23,28)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337223
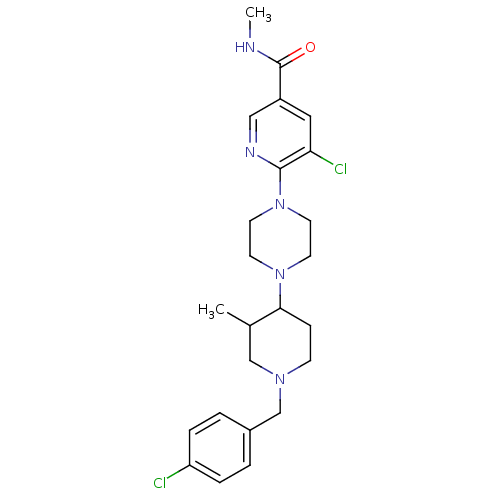
(5-chloro-6-(4-(1-(4-chlorobenzyl)-3-methylpiperidi...)Show SMILES CNC(=O)c1cnc(N2CCN(CC2)C2CCN(Cc3ccc(Cl)cc3)CC2C)c(Cl)c1 Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-29(16-18-3-5-20(25)6-4-18)8-7-22(17)30-9-11-31(12-10-30)23-21(26)13-19(14-28-23)24(32)27-2/h3-6,13-14,17,22H,7-12,15-16H2,1-2H3,(H,27,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337231
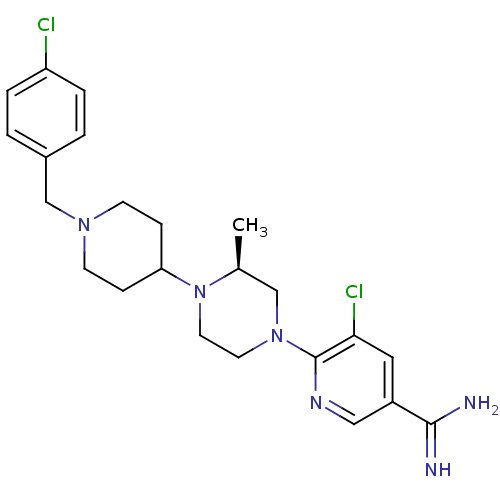
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(N)=N |r| Show InChI InChI=1S/C23H30Cl2N6/c1-16-14-30(23-21(25)12-18(13-28-23)22(26)27)10-11-31(16)20-6-8-29(9-7-20)15-17-2-4-19(24)5-3-17/h2-5,12-13,16,20H,6-11,14-15H2,1H3,(H3,26,27)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337232

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=N)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C25H34Cl2N6/c1-3-29-24(28)20-14-23(27)25(30-15-20)32-12-13-33(18(2)16-32)22-8-10-31(11-9-22)17-19-4-6-21(26)7-5-19/h4-7,14-15,18,22H,3,8-13,16-17H2,1-2H3,(H2,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337217

(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES Fc1ccc(CNC(=O)c2cnc(N3CCN(CC3)C3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)c2)cc1F Show InChI InChI=1S/C29H31Cl2F2N5O/c30-23-4-1-20(2-5-23)19-36-9-7-24(8-10-36)37-11-13-38(14-12-37)28-25(31)16-22(18-34-28)29(39)35-17-21-3-6-26(32)27(33)15-21/h1-6,15-16,18,24H,7-14,17,19H2,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337260

((S)-5-chloro-N-methyl-6-(3-methyl-4-(1-(4-methylbe...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(C)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C25H34ClN5O/c1-18-4-6-20(7-5-18)17-29-10-8-22(9-11-29)31-13-12-30(16-19(31)2)24-23(26)14-21(15-28-24)25(32)27-3/h4-7,14-15,19,22H,8-13,16-17H2,1-3H3,(H,27,32)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337217

(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES Fc1ccc(CNC(=O)c2cnc(N3CCN(CC3)C3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)c2)cc1F Show InChI InChI=1S/C29H31Cl2F2N5O/c30-23-4-1-20(2-5-23)19-36-9-7-24(8-10-36)37-11-13-38(14-12-37)28-25(31)16-22(18-34-28)29(39)35-17-21-3-6-26(32)27(33)15-21/h1-6,15-16,18,24H,7-14,17,19H2,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337245

(CHEMBL1681877 | methyl 4-(3-chloro-5-(methylcarbam...)Show SMILES CNC(=O)c1cnc(N2CCN(C3CCN(Cc4ccc(Cl)cc4)CC3)C(C2)C(=O)OC)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N5O3/c1-28-24(33)18-13-21(27)23(29-14-18)31-11-12-32(22(16-31)25(34)35-2)20-7-9-30(10-8-20)15-17-3-5-19(26)6-4-17/h3-6,13-14,20,22H,7-12,15-16H2,1-2H3,(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337214
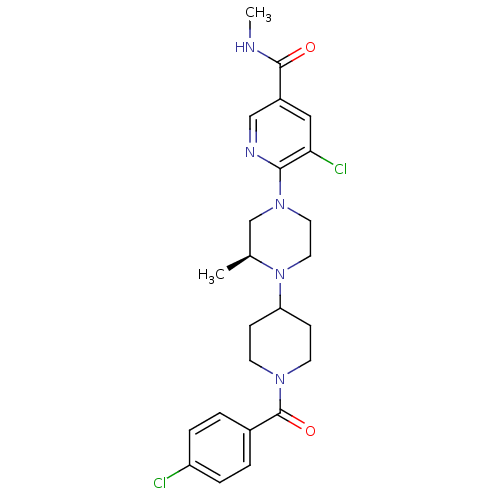
((S)-5-chloro-6-(4-(1-(4-chlorobenzoyl)piperidin-4-...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(=O)c2ccc(Cl)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C24H29Cl2N5O2/c1-16-15-30(22-21(26)13-18(14-28-22)23(32)27-2)11-12-31(16)20-7-9-29(10-8-20)24(33)17-3-5-19(25)6-4-17/h3-6,13-14,16,20H,7-12,15H2,1-2H3,(H,27,32)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337221

((3R)-4-(4-(4-(3,4-dimethylphenyl)but-1-en-2-yl)-2-...)Show SMILES C[C@@H]1CN(CCN1c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1)C1CCN(Cc2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C30H33Cl4N5O/c1-20-18-38(25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)12-13-39(20)29-28(34)15-23(17-35-29)30(40)36-16-22-4-7-26(32)27(33)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337243
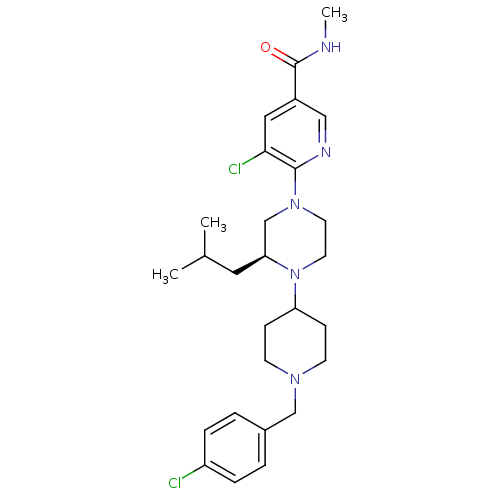
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](CC(C)C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C27H37Cl2N5O/c1-19(2)14-24-18-33(26-25(29)15-21(16-31-26)27(35)30-3)12-13-34(24)23-8-10-32(11-9-23)17-20-4-6-22(28)7-5-20/h4-7,15-16,19,23-24H,8-14,17-18H2,1-3H3,(H,30,35)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337229

(5-chloro-6-(4-(4-(4-chlorobenzyl)piperazin-1-yl)-3...)Show SMILES CNC(=O)c1cnc(N2CCC(C(C)C2)N2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-31(23-21(26)13-19(14-28-23)24(32)27-2)8-7-22(17)30-11-9-29(10-12-30)16-18-3-5-20(25)6-4-18/h3-6,13-14,17,22H,7-12,15-16H2,1-2H3,(H,27,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337230
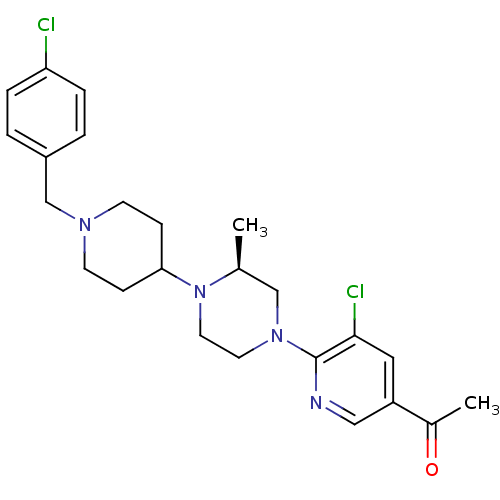
((S)-1-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(C)=O |r| Show InChI InChI=1S/C24H30Cl2N4O/c1-17-15-29(24-23(26)13-20(14-27-24)18(2)31)11-12-30(17)22-7-9-28(10-8-22)16-19-3-5-21(25)6-4-19/h3-6,13-14,17,22H,7-12,15-16H2,1-2H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301346
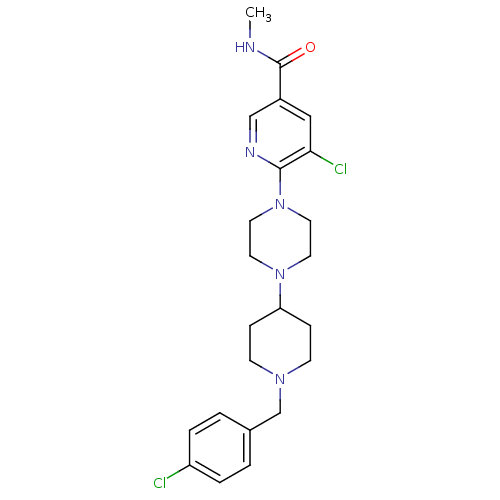
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES CNC(=O)c1cnc(N2CCN(CC2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 Show InChI InChI=1S/C23H29Cl2N5O/c1-26-23(31)18-14-21(25)22(27-15-18)30-12-10-29(11-13-30)20-6-8-28(9-7-20)16-17-2-4-19(24)5-3-17/h2-5,14-15,20H,6-13,16H2,1H3,(H,26,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337241

((S)-azetidin-1-yl(5-chloro-6-(4-(1-(4-chlorobenzyl...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)N1CCC1 |r| Show InChI InChI=1S/C26H33Cl2N5O/c1-19-17-32(25-24(28)15-21(16-29-25)26(34)31-9-2-10-31)13-14-33(19)23-7-11-30(12-8-23)18-20-3-5-22(27)6-4-20/h3-6,15-16,19,23H,2,7-14,17-18H2,1H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337244
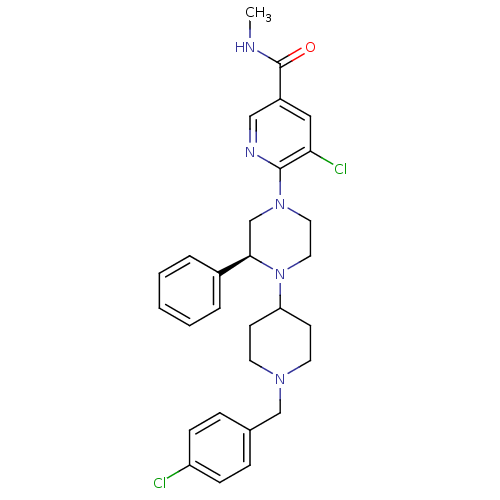
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CNC(=O)c1cnc(N2CCN(C3CCN(Cc4ccc(Cl)cc4)CC3)[C@H](C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C29H33Cl2N5O/c1-32-29(37)23-17-26(31)28(33-18-23)35-15-16-36(27(20-35)22-5-3-2-4-6-22)25-11-13-34(14-12-25)19-21-7-9-24(30)10-8-21/h2-10,17-18,25,27H,11-16,19-20H2,1H3,(H,32,37)/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337240
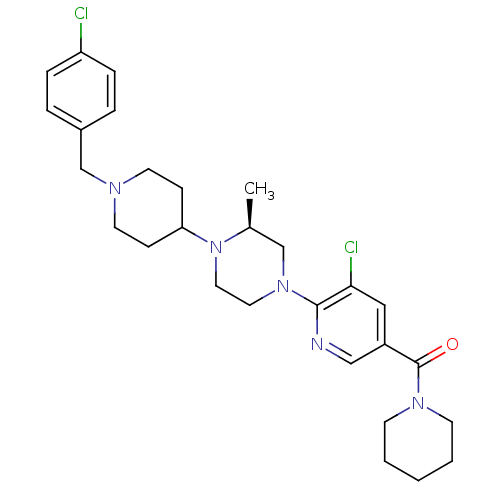
((S)-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C28H37Cl2N5O/c1-21-19-34(27-26(30)17-23(18-31-27)28(36)33-11-3-2-4-12-33)15-16-35(21)25-9-13-32(14-10-25)20-22-5-7-24(29)8-6-22/h5-8,17-18,21,25H,2-4,9-16,19-20H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337246
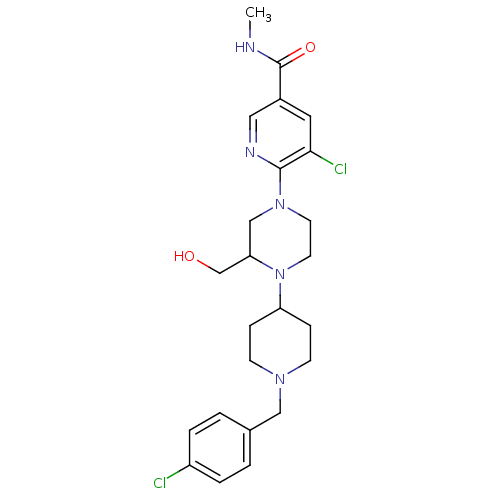
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)-3...)Show SMILES CNC(=O)c1cnc(N2CCN(C(CO)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 Show InChI InChI=1S/C24H31Cl2N5O2/c1-27-24(33)18-12-22(26)23(28-13-18)30-10-11-31(21(15-30)16-32)20-6-8-29(9-7-20)14-17-2-4-19(25)5-3-17/h2-5,12-13,20-21,32H,6-11,14-16H2,1H3,(H,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337225

(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)-3...)Show SMILES Fc1ccc(CNC(=O)c2cnc(N3CCN(C4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)C3)c(Cl)c2)cc1F Show InChI InChI=1S/C29H29Cl2F2N5O2/c30-22-4-1-19(2-5-22)17-36-9-7-23(8-10-36)38-12-11-37(18-27(38)39)28-24(31)14-21(16-34-28)29(40)35-15-20-3-6-25(32)26(33)13-20/h1-6,13-14,16,23H,7-12,15,17-18H2,(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337256
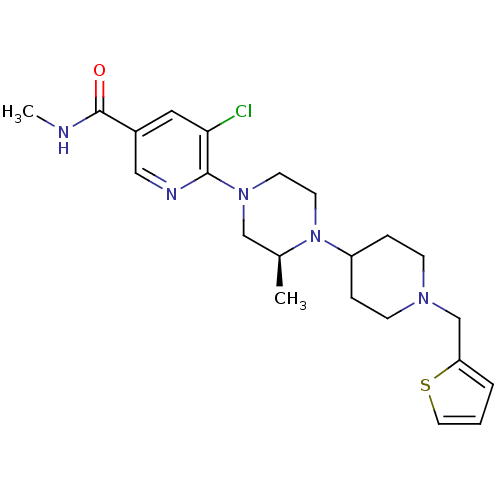
((S)-5-chloro-N-methyl-6-(3-methyl-4-(1-(thiophen-2...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3cccs3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C22H30ClN5OS/c1-16-14-27(21-20(23)12-17(13-25-21)22(29)24-2)9-10-28(16)18-5-7-26(8-6-18)15-19-4-3-11-30-19/h3-4,11-13,16,18H,5-10,14-15H2,1-2H3,(H,24,29)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337222

(6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)-2-methylpi...)Show SMILES CC1CN(CCN1c1ccc(cn1)C(=O)NCc1ccc(F)c(F)c1)C1CCN(Cc2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C30H34ClF2N5O/c1-21-19-37(26-10-12-36(13-11-26)20-22-2-6-25(31)7-3-22)14-15-38(21)29-9-5-24(18-34-29)30(39)35-17-23-4-8-27(32)28(33)16-23/h2-9,16,18,21,26H,10-15,17,19-20H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337247
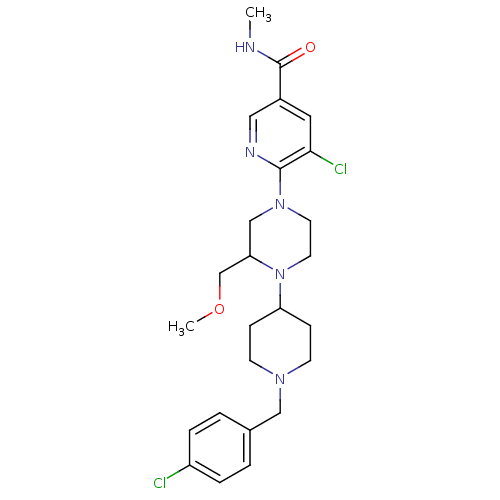
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)-3...)Show SMILES CNC(=O)c1cnc(N2CCN(C(COC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 Show InChI InChI=1S/C25H33Cl2N5O2/c1-28-25(33)19-13-23(27)24(29-14-19)31-11-12-32(22(16-31)17-34-2)21-7-9-30(10-8-21)15-18-3-5-20(26)6-4-18/h3-6,13-14,21-22H,7-12,15-17H2,1-2H3,(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301332
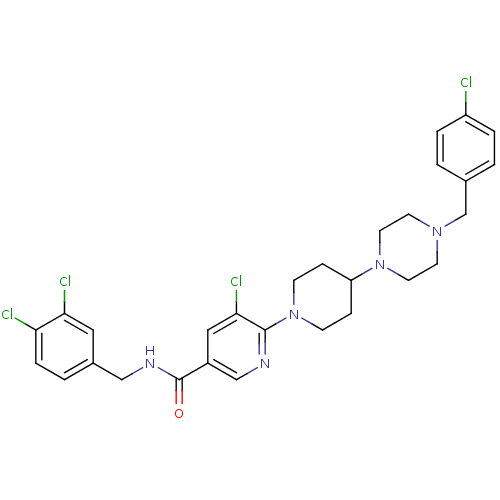
(5-chloro-6-(4-(4-(4-chlorobenzyl)piperazin-1-yl)pi...)Show SMILES Clc1ccc(CN2CCN(CC2)C2CCN(CC2)c2ncc(cc2Cl)C(=O)NCc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C29H31Cl4N5O/c30-23-4-1-20(2-5-23)19-36-11-13-37(14-12-36)24-7-9-38(10-8-24)28-27(33)16-22(18-34-28)29(39)35-17-21-3-6-25(31)26(32)15-21/h1-6,15-16,18,24H,7-14,17,19H2,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337226
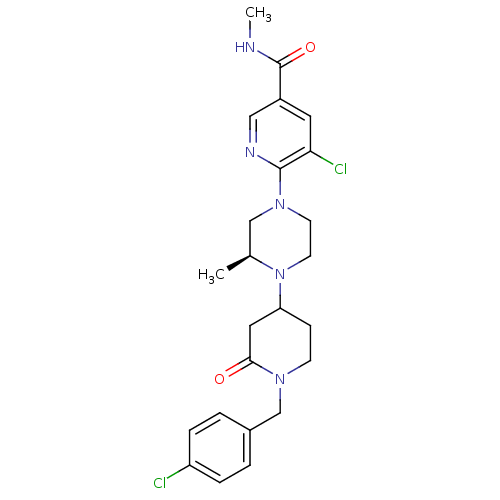
(5-chloro-6-((3S)-4-(1-(4-chlorobenzyl)-2-oxopiperi...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)C(=O)C2)c(Cl)c1 |r| Show InChI InChI=1S/C24H29Cl2N5O2/c1-16-14-30(23-21(26)11-18(13-28-23)24(33)27-2)9-10-31(16)20-7-8-29(22(32)12-20)15-17-3-5-19(25)6-4-17/h3-6,11,13,16,20H,7-10,12,14-15H2,1-2H3,(H,27,33)/t16-,20?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337228
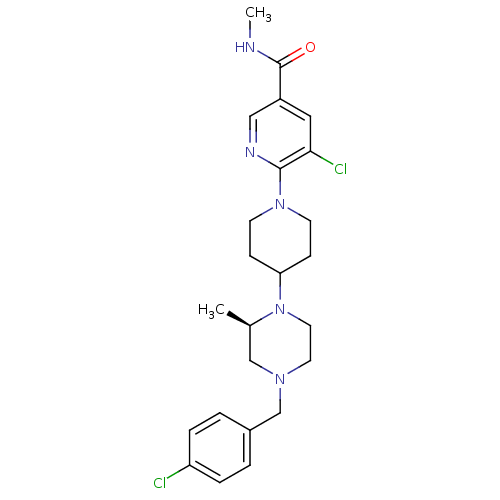
((R)-5-chloro-6-(4-(4-(4-chlorobenzyl)-2-methylpipe...)Show SMILES CNC(=O)c1cnc(N2CCC(CC2)N2CCN(Cc3ccc(Cl)cc3)C[C@H]2C)c(Cl)c1 |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-29(16-18-3-5-20(25)6-4-18)11-12-31(17)21-7-9-30(10-8-21)23-22(26)13-19(14-28-23)24(32)27-2/h3-6,13-14,17,21H,7-12,15-16H2,1-2H3,(H,27,32)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337258
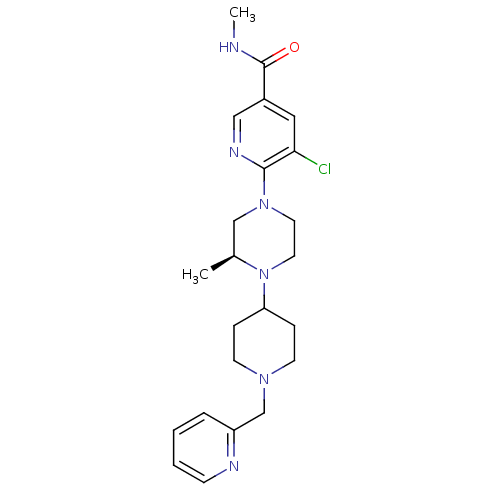
((S)-5-chloro-N-methyl-6-(3-methyl-4-(1-(pyridin-2-...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccccn3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C23H31ClN6O/c1-17-15-29(22-21(24)13-18(14-27-22)23(31)25-2)11-12-30(17)20-6-9-28(10-7-20)16-19-5-3-4-8-26-19/h3-5,8,13-14,17,20H,6-7,9-12,15-16H2,1-2H3,(H,25,31)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337257

((S)-6-(4-(1-((1H-pyrrol-2-yl)methyl)piperidin-4-yl...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc[nH]3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C22H31ClN6O/c1-16-14-28(21-20(23)12-17(13-26-21)22(30)24-2)10-11-29(16)19-5-8-27(9-6-19)15-18-4-3-7-25-18/h3-4,7,12-13,16,19,25H,5-6,8-11,14-15H2,1-2H3,(H,24,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337255

((S)-5-chloro-6-(4-(1-(furan-2-ylmethyl)piperidin-4...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccco3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C22H30ClN5O2/c1-16-14-27(21-20(23)12-17(13-25-21)22(29)24-2)9-10-28(16)18-5-7-26(8-6-18)15-19-4-3-11-30-19/h3-4,11-13,16,18H,5-10,14-15H2,1-2H3,(H,24,29)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337249
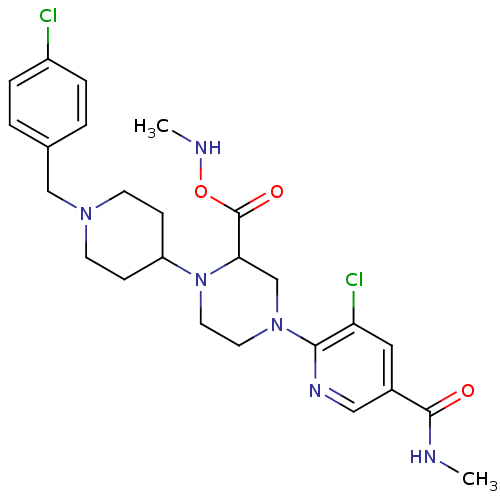
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)-3...)Show SMILES CNOC(=O)C1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NC Show InChI InChI=1S/C25H32Cl2N6O3/c1-28-24(34)18-13-21(27)23(30-14-18)32-11-12-33(22(16-32)25(35)36-29-2)20-7-9-31(10-8-20)15-17-3-5-19(26)6-4-17/h3-6,13-14,20,22,29H,7-12,15-16H2,1-2H3,(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337259
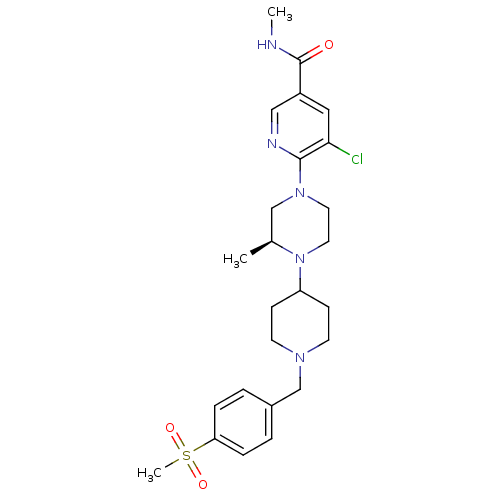
((S)-5-chloro-N-methyl-6-(3-methyl-4-(1-(4-(methyls...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(cc3)S(C)(=O)=O)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C25H34ClN5O3S/c1-18-16-30(24-23(26)14-20(15-28-24)25(32)27-2)12-13-31(18)21-8-10-29(11-9-21)17-19-4-6-22(7-5-19)35(3,33)34/h4-7,14-15,18,21H,8-13,16-17H2,1-3H3,(H,27,32)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337216

((S)-5-chloro-6-(4-(1-(7-chloroquinoline-4-carbonyl...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(=O)c2ccnc3cc(Cl)ccc23)c(Cl)c1 |r| Show InChI InChI=1S/C27H30Cl2N6O2/c1-17-16-34(25-23(29)13-18(15-32-25)26(36)30-2)11-12-35(17)20-6-9-33(10-7-20)27(37)22-5-8-31-24-14-19(28)3-4-21(22)24/h3-5,8,13-15,17,20H,6-7,9-12,16H2,1-2H3,(H,30,36)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337215
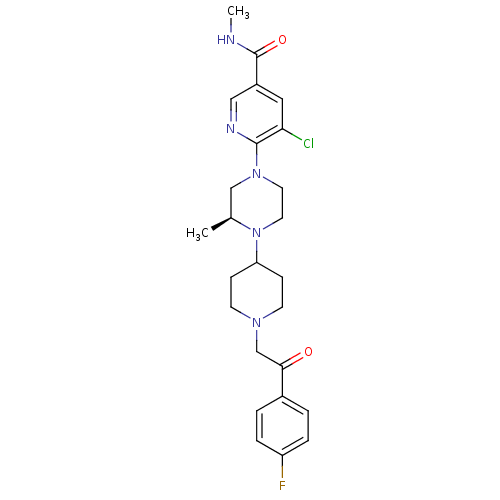
((S)-5-chloro-6-(4-(1-(2-(4-fluorophenyl)-2-oxoethy...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC(=O)c3ccc(F)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C25H31ClFN5O2/c1-17-15-31(24-22(26)13-19(14-29-24)25(34)28-2)11-12-32(17)21-7-9-30(10-8-21)16-23(33)18-3-5-20(27)6-4-18/h3-6,13-14,17,21H,7-12,15-16H2,1-2H3,(H,28,34)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337227
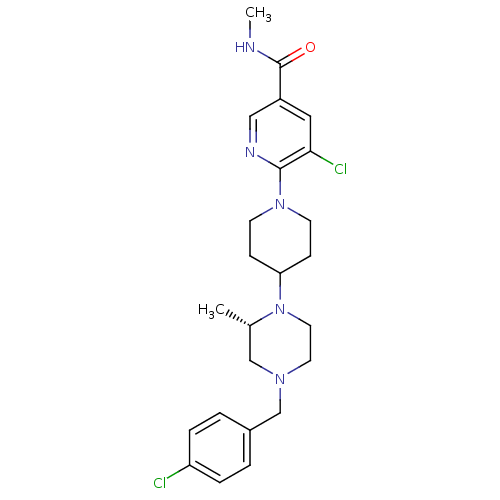
((S)-5-chloro-6-(4-(4-(4-chlorobenzyl)-2-methylpipe...)Show SMILES CNC(=O)c1cnc(N2CCC(CC2)N2CCN(Cc3ccc(Cl)cc3)C[C@@H]2C)c(Cl)c1 |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-29(16-18-3-5-20(25)6-4-18)11-12-31(17)21-7-9-30(10-8-21)23-22(26)13-19(14-28-23)24(32)27-2/h3-6,13-14,17,21H,7-12,15-16H2,1-2H3,(H,27,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337248

(4-(3-chloro-5-(methylcarbamoyl)pyridin-2-yl)-1-(1-...)Show SMILES CNC(=O)c1cnc(N2CCN(C3CCN(Cc4ccc(Cl)cc4)CC3)C(C2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C24H29Cl2N5O3/c1-27-23(32)17-12-20(26)22(28-13-17)30-10-11-31(21(15-30)24(33)34)19-6-8-29(9-7-19)14-16-2-4-18(25)5-3-16/h2-5,12-13,19,21H,6-11,14-15H2,1H3,(H,27,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data