 Found 36 hits of Enzyme Inhibition Constant Data
Found 36 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343334
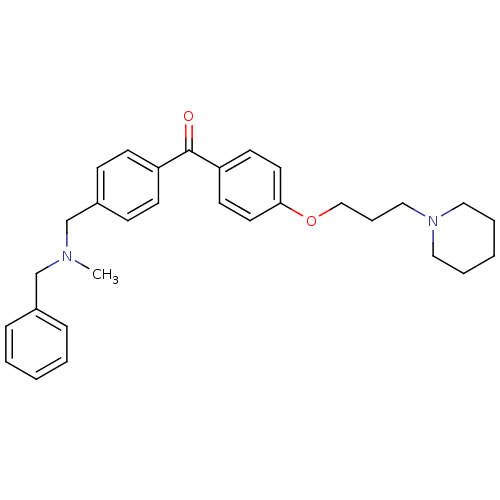
(CHEMBL1773483 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H36N2O2/c1-31(23-25-9-4-2-5-10-25)24-26-11-13-27(14-12-26)30(33)28-15-17-29(18-16-28)34-22-8-21-32-19-6-3-7-20-32/h2,4-5,9-18H,3,6-8,19-24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343333
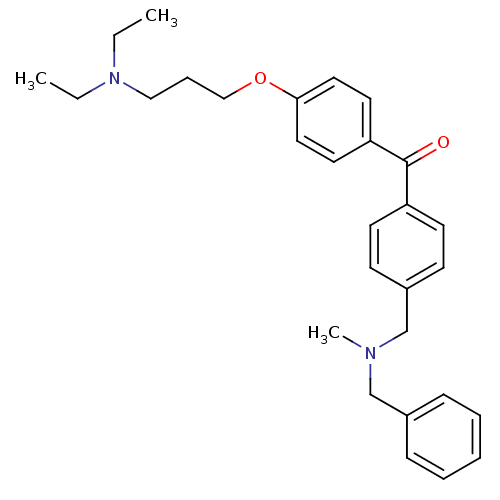
(CHEMBL1773482 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C29H36N2O2/c1-4-31(5-2)20-9-21-33-28-18-16-27(17-19-28)29(32)26-14-12-25(13-15-26)23-30(3)22-24-10-7-6-8-11-24/h6-8,10-19H,4-5,9,20-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343336
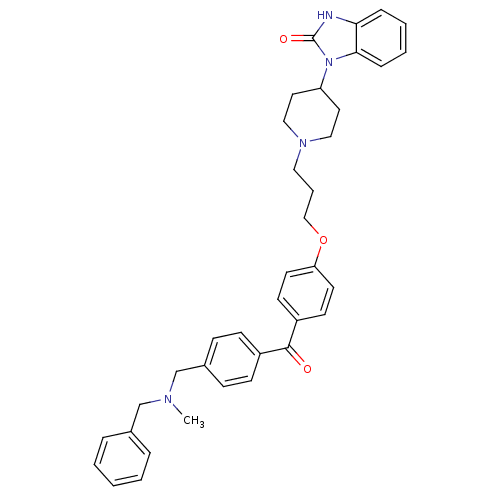
(1-{1-[3-(4-{4-[(Benzylmethylamino)methyl]benzoyl}p...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCN2CCC(CC2)n2c3ccccc3[nH]c2=O)cc1 Show InChI InChI=1S/C37H40N4O3/c1-39(26-28-8-3-2-4-9-28)27-29-12-14-30(15-13-29)36(42)31-16-18-33(19-17-31)44-25-7-22-40-23-20-32(21-24-40)41-35-11-6-5-10-34(35)38-37(41)43/h2-6,8-19,32H,7,20-27H2,1H3,(H,38,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343335

(CHEMBL1773484 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCN2CCOCC2)cc1 Show InChI InChI=1S/C29H34N2O3/c1-30(22-24-6-3-2-4-7-24)23-25-8-10-26(11-9-25)29(32)27-12-14-28(15-13-27)34-19-5-16-31-17-20-33-21-18-31/h2-4,6-15H,5,16-23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343342
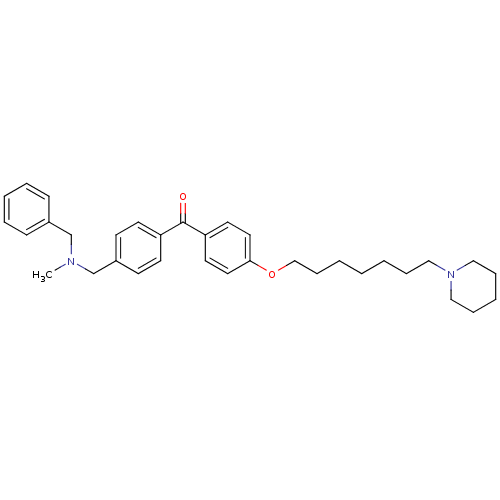
(CHEMBL1773491 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C34H44N2O2/c1-35(27-29-13-7-5-8-14-29)28-30-15-17-31(18-16-30)34(37)32-19-21-33(22-20-32)38-26-12-4-2-3-9-23-36-24-10-6-11-25-36/h5,7-8,13-22H,2-4,6,9-12,23-28H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343343

(CHEMBL1773492 | {4-[(Benzylmethylaminomethyl]pheny...)Show SMILES CCN(CC)CCc1ccc(Oc2ccc(cc2)C(=O)c2ccc(CN(C)Cc3ccccc3)cc2)cc1 Show InChI InChI=1S/C34H38N2O2/c1-4-36(5-2)24-23-27-13-19-32(20-14-27)38-33-21-17-31(18-22-33)34(37)30-15-11-29(12-16-30)26-35(3)25-28-9-7-6-8-10-28/h6-22H,4-5,23-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343338

(CHEMBL1773487 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H40N2O2/c1-33(25-27-11-5-2-6-12-27)26-28-13-15-29(16-14-28)32(35)30-17-19-31(20-18-30)36-24-10-4-9-23-34-21-7-3-8-22-34/h2,5-6,11-20H,3-4,7-10,21-26H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343337
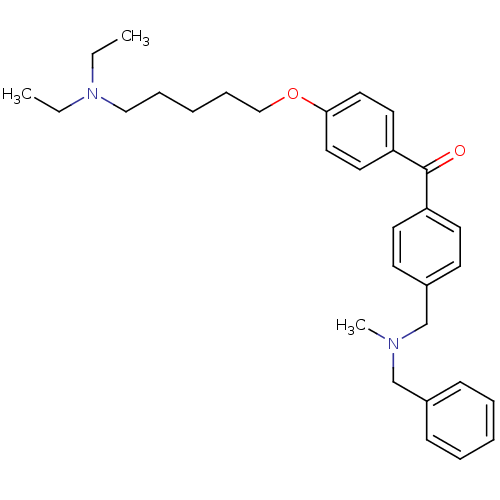
(CHEMBL1773486 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C31H40N2O2/c1-4-33(5-2)22-10-7-11-23-35-30-20-18-29(19-21-30)31(34)28-16-14-27(15-17-28)25-32(3)24-26-12-8-6-9-13-26/h6,8-9,12-21H,4-5,7,10-11,22-25H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343340

(CHEMBL1773489 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C33H42N2O2/c1-34(26-28-12-6-4-7-13-28)27-29-14-16-30(17-15-29)33(36)31-18-20-32(21-19-31)37-25-11-3-2-8-22-35-23-9-5-10-24-35/h4,6-7,12-21H,2-3,5,8-11,22-27H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343329

(CHEMBL1773478 | N-(4-{4-[(Benzylmethylamino)methyl...)Show SMILES CCN(CC)CC(=O)Nc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C28H33N3O2/c1-4-31(5-2)21-27(32)29-26-17-15-25(16-18-26)28(33)24-13-11-23(12-14-24)20-30(3)19-22-9-7-6-8-10-22/h6-18H,4-5,19-21H2,1-3H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343332
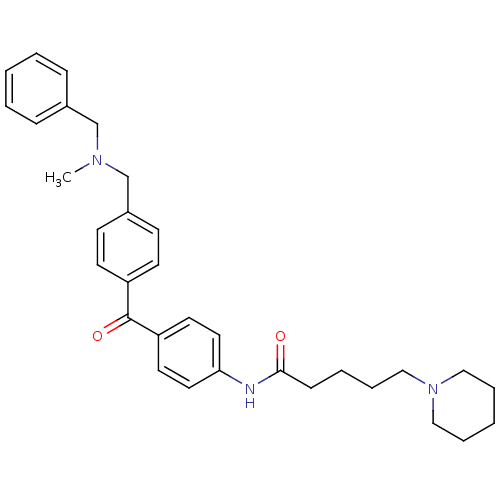
(5-Piperidin-1-ylpentanoicacid(4-{4-[(benzylmethyla...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(NC(=O)CCCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H39N3O2/c1-34(24-26-10-4-2-5-11-26)25-27-13-15-28(16-14-27)32(37)29-17-19-30(20-18-29)33-31(36)12-6-9-23-35-21-7-3-8-22-35/h2,4-5,10-11,13-20H,3,6-9,12,21-25H2,1H3,(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343339

(CHEMBL1773488 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCCCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C32H42N2O2/c1-4-34(5-2)23-11-6-7-12-24-36-31-21-19-30(20-22-31)32(35)29-17-15-28(16-18-29)26-33(3)25-27-13-9-8-10-14-27/h8-10,13-22H,4-7,11-12,23-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343330

(CHEMBL1773479 | N-(4-{4-[(Benzylmethylamino)methyl...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(NC(=O)CN2CCCCC2)cc1 Show InChI InChI=1S/C29H33N3O2/c1-31(20-23-8-4-2-5-9-23)21-24-10-12-25(13-11-24)29(34)26-14-16-27(17-15-26)30-28(33)22-32-18-6-3-7-19-32/h2,4-5,8-17H,3,6-7,18-22H2,1H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343331

(CHEMBL1773480 | N-(4-{4-[(Benzylmethylamino)methyl...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(NC(=O)CN2CCOCC2)cc1 Show InChI InChI=1S/C28H31N3O3/c1-30(19-22-5-3-2-4-6-22)20-23-7-9-24(10-8-23)28(33)25-11-13-26(14-12-25)29-27(32)21-31-15-17-34-18-16-31/h2-14H,15-21H2,1H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50343341

(CHEMBL1773490 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCCCCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C33H44N2O2/c1-4-35(5-2)24-12-7-6-8-13-25-37-32-22-20-31(21-23-32)33(36)30-18-16-29(17-19-30)27-34(3)26-28-14-10-9-11-15-28/h9-11,14-23H,4-8,12-13,24-27H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50249616

(CHEMBL514966 | {4-[(Benzylmethylamino)methyl]pheny...)Show InChI InChI=1S/C23H23NO2/c1-24(16-18-6-4-3-5-7-18)17-19-8-10-20(11-9-19)23(25)21-12-14-22(26-2)15-13-21/h3-15H,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343340

(CHEMBL1773489 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C33H42N2O2/c1-34(26-28-12-6-4-7-13-28)27-29-14-16-30(17-15-29)33(36)31-18-20-32(21-19-31)37-25-11-3-2-8-22-35-23-9-5-10-24-35/h4,6-7,12-21H,2-3,5,8-11,22-27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50249616

(CHEMBL514966 | {4-[(Benzylmethylamino)methyl]pheny...)Show InChI InChI=1S/C23H23NO2/c1-24(16-18-6-4-3-5-7-18)17-19-8-10-20(11-9-19)23(25)21-12-14-22(26-2)15-13-21/h3-15H,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343338

(CHEMBL1773487 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H40N2O2/c1-33(25-27-11-5-2-6-12-27)26-28-13-15-29(16-14-28)32(35)30-17-19-31(20-18-30)36-24-10-4-9-23-34-21-7-3-8-22-34/h2,5-6,11-20H,3-4,7-10,21-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343334

(CHEMBL1773483 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H36N2O2/c1-31(23-25-9-4-2-5-10-25)24-26-11-13-27(14-12-26)30(33)28-15-17-29(18-16-28)34-22-8-21-32-19-6-3-7-20-32/h2,4-5,9-18H,3,6-8,19-24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM31904
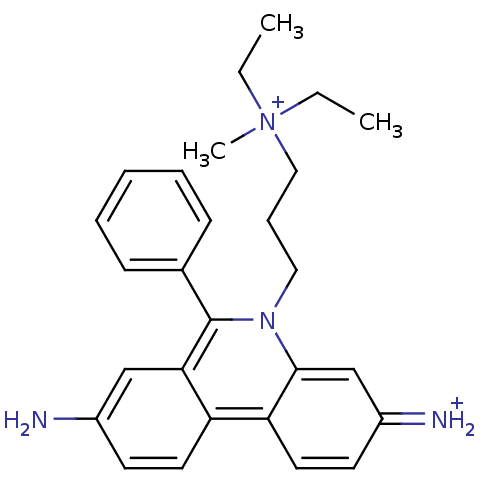
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343335

(CHEMBL1773484 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCN2CCOCC2)cc1 Show InChI InChI=1S/C29H34N2O3/c1-30(22-24-6-3-2-4-7-24)23-25-8-10-26(11-9-25)29(32)27-12-14-28(15-13-27)34-19-5-16-31-17-20-33-21-18-31/h2-4,6-15H,5,16-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343339

(CHEMBL1773488 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCCCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C32H42N2O2/c1-4-34(5-2)23-11-6-7-12-24-36-31-21-19-30(20-22-31)32(35)29-17-15-28(16-18-29)26-33(3)25-27-13-9-8-10-14-27/h8-10,13-22H,4-7,11-12,23-26H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343341

(CHEMBL1773490 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCCCCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C33H44N2O2/c1-4-35(5-2)24-12-7-6-8-13-25-37-32-22-20-31(21-23-32)33(36)30-18-16-29(17-19-30)27-34(3)26-28-14-10-9-11-15-28/h9-11,14-23H,4-8,12-13,24-27H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343342

(CHEMBL1773491 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C34H44N2O2/c1-35(27-29-13-7-5-8-14-29)28-30-15-17-31(18-16-30)34(37)32-19-21-33(22-20-32)38-26-12-4-2-3-9-23-36-24-10-6-11-25-36/h5,7-8,13-22H,2-4,6,9-12,23-28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM31904

(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate by Ellman's assay |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343337

(CHEMBL1773486 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C31H40N2O2/c1-4-33(5-2)22-10-7-11-23-35-30-20-18-29(19-21-30)31(34)28-16-14-27(15-17-28)25-32(3)24-26-12-8-6-9-13-26/h6,8-9,12-21H,4-5,7,10-11,22-25H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343333

(CHEMBL1773482 | {4-[(Benzylmethylamino)methyl]phen...)Show SMILES CCN(CC)CCCOc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C29H36N2O2/c1-4-31(5-2)20-9-21-33-28-18-16-27(17-19-28)29(32)26-14-12-25(13-15-26)23-30(3)22-24-10-7-6-8-11-24/h6-8,10-19H,4-5,9,20-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343336

(1-{1-[3-(4-{4-[(Benzylmethylamino)methyl]benzoyl}p...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(OCCCN2CCC(CC2)n2c3ccccc3[nH]c2=O)cc1 Show InChI InChI=1S/C37H40N4O3/c1-39(26-28-8-3-2-4-9-28)27-29-12-14-30(15-13-29)36(42)31-16-18-33(19-17-31)44-25-7-22-40-23-20-32(21-24-40)41-35-11-6-5-10-34(35)38-37(41)43/h2-6,8-19,32H,7,20-27H2,1H3,(H,38,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343332

(5-Piperidin-1-ylpentanoicacid(4-{4-[(benzylmethyla...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(NC(=O)CCCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H39N3O2/c1-34(24-26-10-4-2-5-11-26)25-27-13-15-28(16-14-27)32(37)29-17-19-30(20-18-29)33-31(36)12-6-9-23-35-21-7-3-8-22-35/h2,4-5,10-11,13-20H,3,6-9,12,21-25H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343329

(CHEMBL1773478 | N-(4-{4-[(Benzylmethylamino)methyl...)Show SMILES CCN(CC)CC(=O)Nc1ccc(cc1)C(=O)c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C28H33N3O2/c1-4-31(5-2)21-27(32)29-26-17-15-25(16-18-26)28(33)24-13-11-23(12-14-24)20-30(3)19-22-9-7-6-8-10-22/h6-18H,4-5,19-21H2,1-3H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343330

(CHEMBL1773479 | N-(4-{4-[(Benzylmethylamino)methyl...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(NC(=O)CN2CCCCC2)cc1 Show InChI InChI=1S/C29H33N3O2/c1-31(20-23-8-4-2-5-9-23)21-24-10-12-25(13-11-24)29(34)26-14-16-27(17-15-26)30-28(33)22-32-18-6-3-7-19-32/h2,4-5,8-17H,3,6-7,18-22H2,1H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343331

(CHEMBL1773480 | N-(4-{4-[(Benzylmethylamino)methyl...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)C(=O)c1ccc(NC(=O)CN2CCOCC2)cc1 Show InChI InChI=1S/C28H31N3O3/c1-30(19-22-5-3-2-4-6-22)20-23-7-9-24(10-8-23)28(33)25-11-13-26(14-12-25)29-27(32)21-31-15-17-34-18-16-31/h2-14H,15-21H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50343343

(CHEMBL1773492 | {4-[(Benzylmethylaminomethyl]pheny...)Show SMILES CCN(CC)CCc1ccc(Oc2ccc(cc2)C(=O)c2ccc(CN(C)Cc3ccccc3)cc2)cc1 Show InChI InChI=1S/C34H38N2O2/c1-4-36(5-2)24-23-27-13-19-32(20-14-27)38-33-21-17-31(18-22-33)34(37)30-15-11-29(12-16-30)26-35(3)25-28-9-7-6-8-10-28/h6-22H,4-5,23-26H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 46: 1682-93 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.019
BindingDB Entry DOI: 10.7270/Q2X92BM2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data