

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342149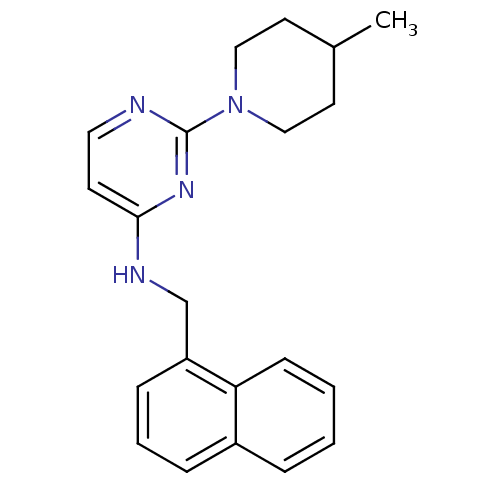 (2-(4-Methylpiperidin-1-yl)-N-(naphth-1-ylmethyl)py...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319981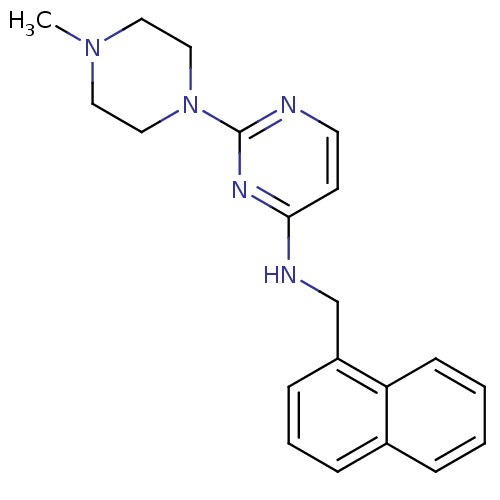 (2-(4-methylpiperazin-1-yl)-N-(naphthalen-1-ylmethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319972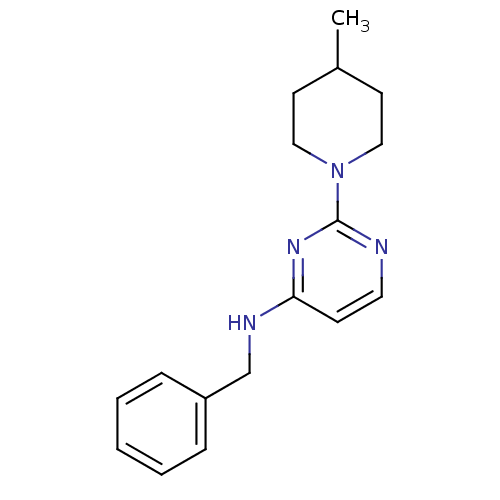 (CHEMBL1082977 | N-benzyl-2-(4-methylpiperidin-1-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342163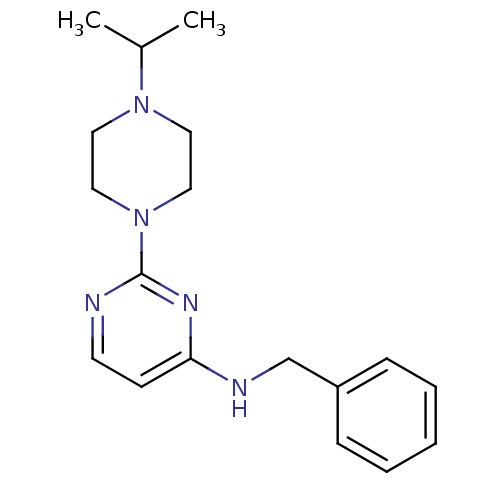 (CHEMBL1766019 | N-Benzyl-2-(4-isopropylpiperazin-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319978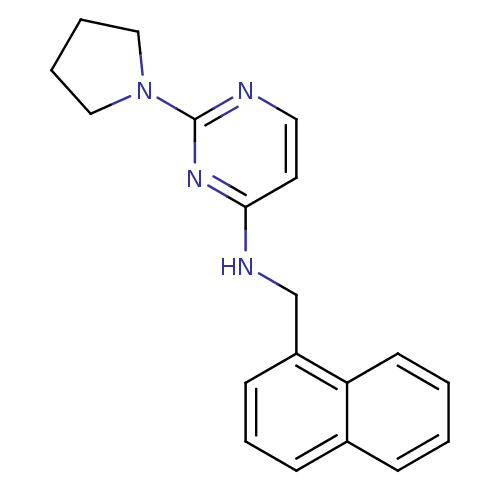 (CHEMBL1084210 | N-(naphthalen-1-ylmethyl)-2-(pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319970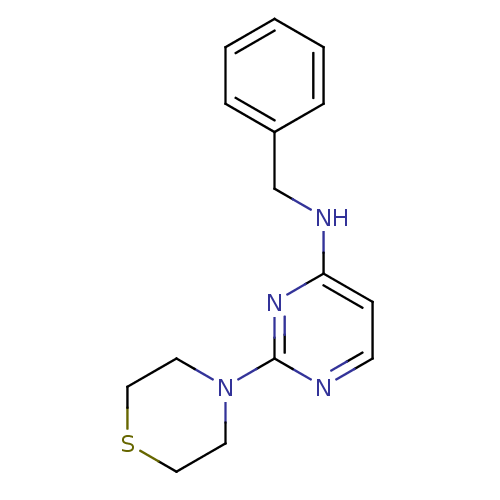 (CHEMBL1086014 | N-benzyl-2-thiomorpholinopyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342162 (CHEMBL1766020 | N-Benzyl-2-(4-isopropylpiperidin-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342156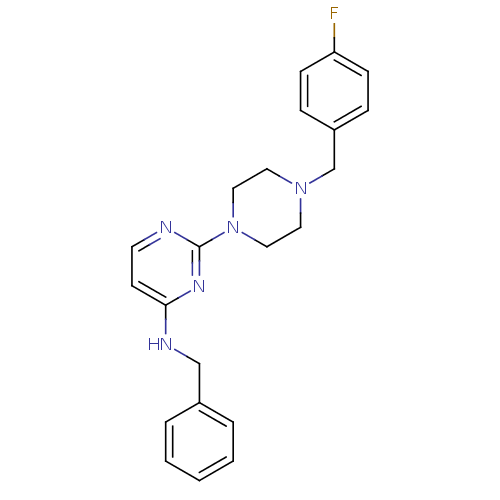 (CHEMBL1766144 | N-Benzyl-2-[4-(4-fluorobenzyl)pipe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342145 (CHEMBL1766018 | N-Benzyl-2-(4-cyclohexylpiperazin-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342154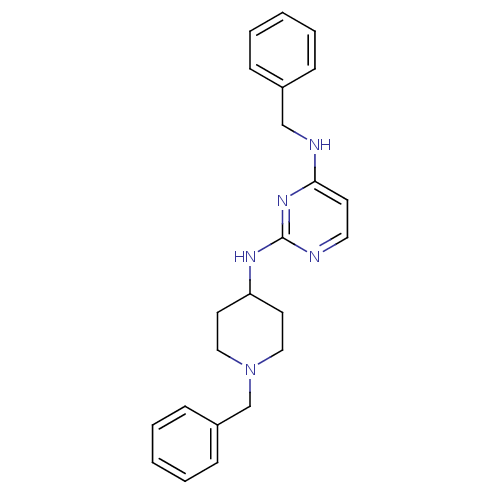 (CHEMBL1766146 | N4-Benzyl-N2-(1-benzylpiperidin-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319968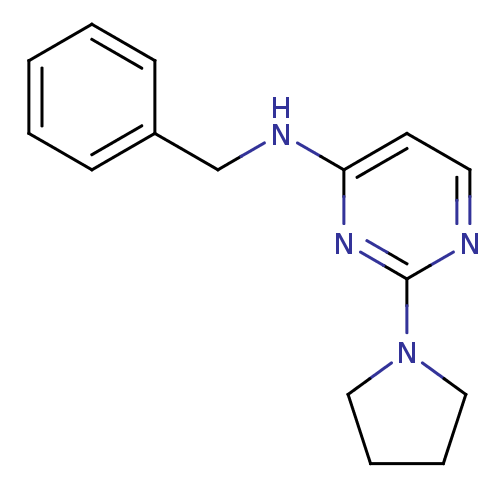 (CHEMBL1085780 | N-benzyl-2-(pyrrolidin-1-yl)pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319977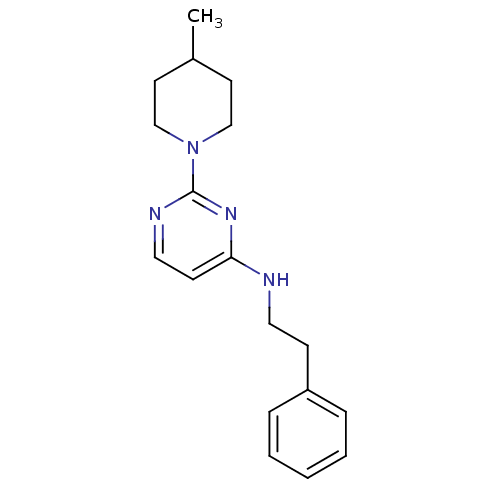 (2-(4-methylpiperidin-1-yl)-N-phenethylpyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319978 (CHEMBL1084210 | N-(naphthalen-1-ylmethyl)-2-(pyrro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319973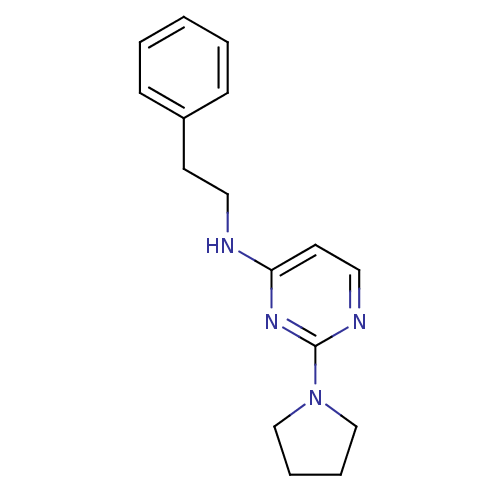 (CHEMBL1082978 | N-phenethyl-2-(pyrrolidin-1-yl)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342158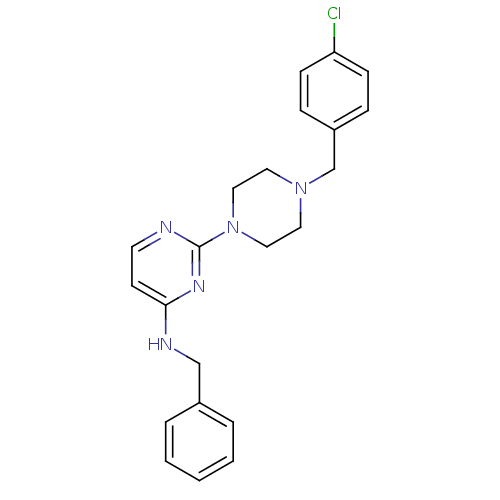 (CHEMBL1766142 | N-Benzyl-2-[4-(4-chlorobenzyl)pipe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342154 (CHEMBL1766146 | N4-Benzyl-N2-(1-benzylpiperidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342153 (4-[4-(benzylamino)pyrimidin-2-yl]thiomorpholine-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319980 (CHEMBL1084212 | N-(naphthalen-1-ylmethyl)-2-thiomo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319973 (CHEMBL1082978 | N-phenethyl-2-(pyrrolidin-1-yl)pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319969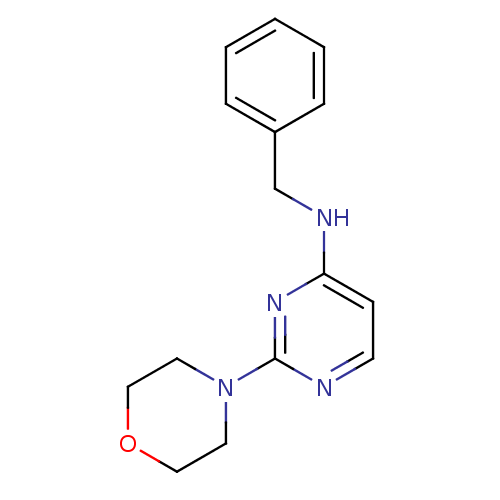 (CHEMBL1085781 | N-benzyl-2-morpholinopyrimidin-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342162 (CHEMBL1766020 | N-Benzyl-2-(4-isopropylpiperidin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342157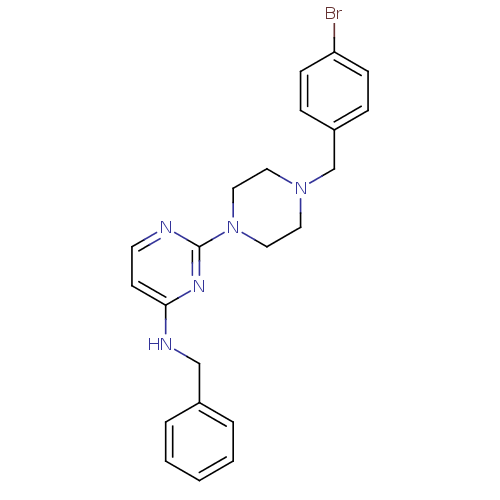 (CHEMBL1766143 | N-Benzyl-2-[4-(4-bromobenzyl)piper...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319979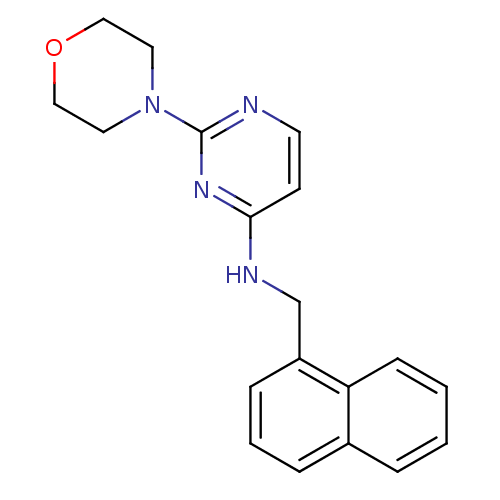 (2-morpholino-N-(naphthalen-1-ylmethyl)pyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342161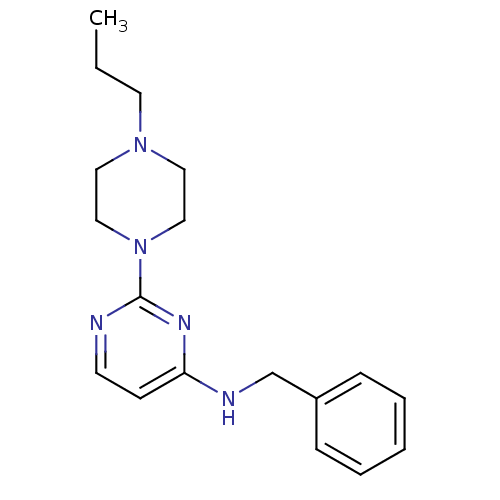 (CHEMBL1766139 | N-Benzyl-2-(4-propylpiperazin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342151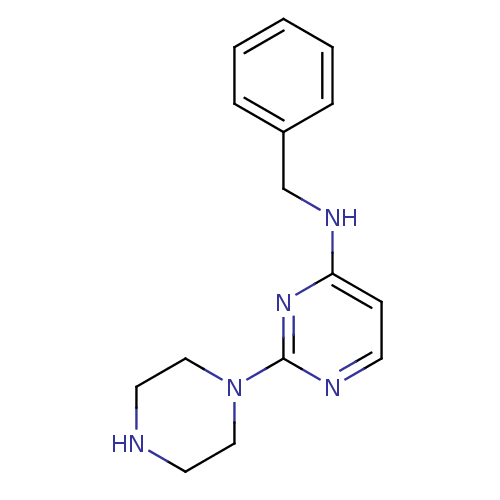 (CHEMBL1766149 | N-benzyl-2-(piperazin-1-yl)pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342147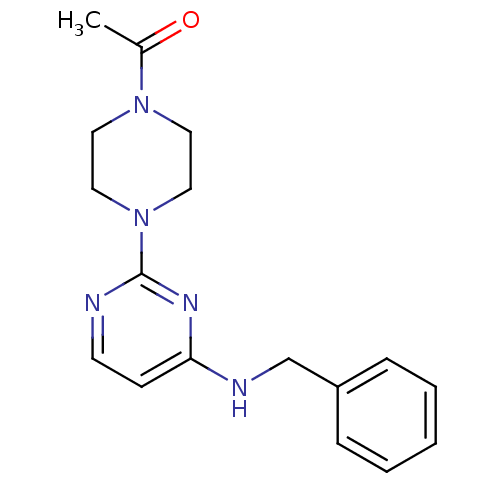 (1-[4-(4-(Benzylamino)pyrimidin-2-yl)piperazin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319981 (2-(4-methylpiperazin-1-yl)-N-(naphthalen-1-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319977 (2-(4-methylpiperidin-1-yl)-N-phenethylpyrimidin-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319972 (CHEMBL1082977 | N-benzyl-2-(4-methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342146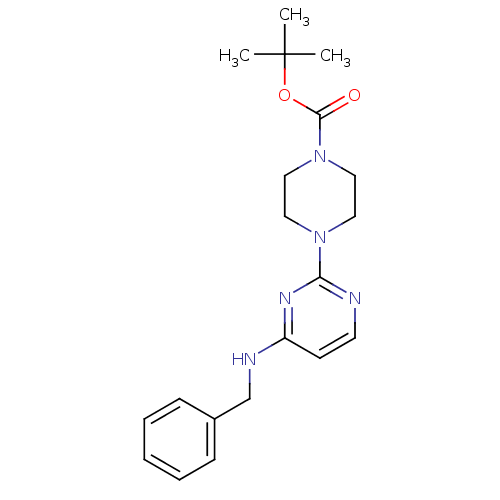 (CHEMBL1766017 | tert-Butyl 4-[4-(Benzylamino)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319974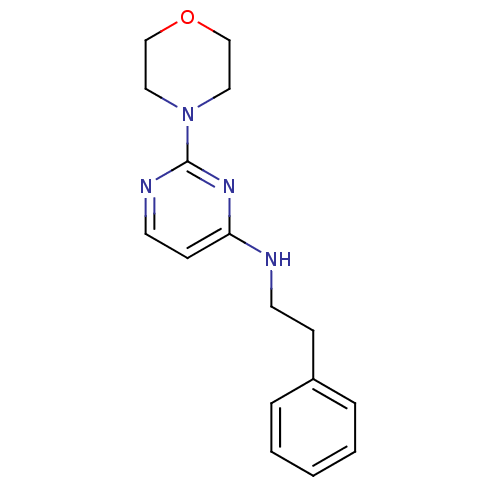 (2-morpholino-N-phenethylpyrimidin-4-amine | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342150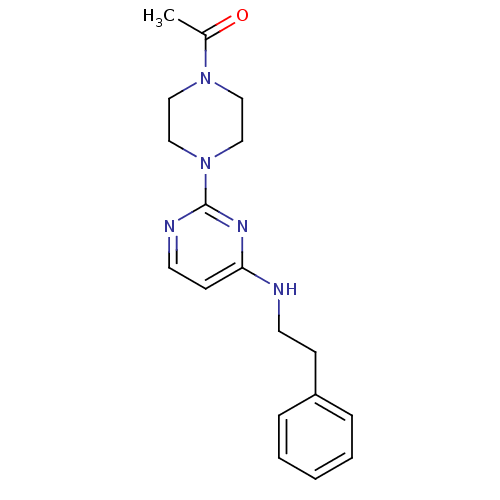 (1-[4-(4-(Phenethylamino)pyrimidin-2-yl)piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342158 (CHEMBL1766142 | N-Benzyl-2-[4-(4-chlorobenzyl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319976 (2-(4-methylpiperazin-1-yl)-N-phenethylpyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342156 (CHEMBL1766144 | N-Benzyl-2-[4-(4-fluorobenzyl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342145 (CHEMBL1766018 | N-Benzyl-2-(4-cyclohexylpiperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319970 (CHEMBL1086014 | N-benzyl-2-thiomorpholinopyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342152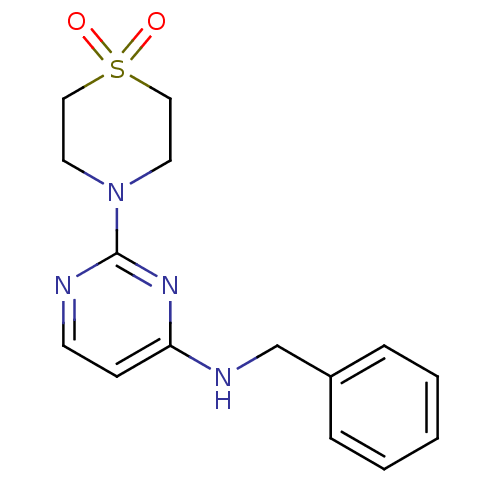 (4-[4-(benzylamino)pyrimidin-2-yl]thiomorpholine-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319971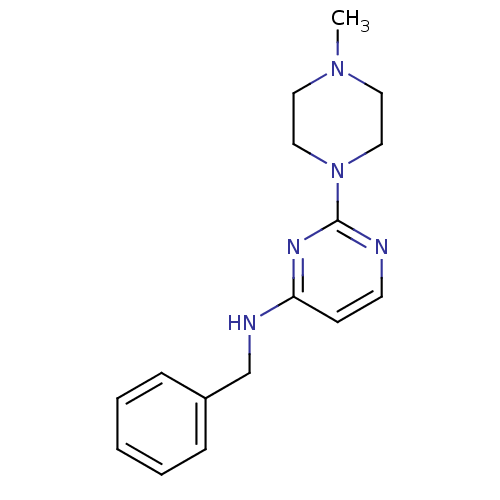 (CHEMBL1084808 | N-benzyl-2-(4-methylpiperazin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342163 (CHEMBL1766019 | N-Benzyl-2-(4-isopropylpiperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342157 (CHEMBL1766143 | N-Benzyl-2-[4-(4-bromobenzyl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342149 (2-(4-Methylpiperidin-1-yl)-N-(naphth-1-ylmethyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50319975 (CHEMBL1082979 | N-phenethyl-2-thiomorpholinopyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319968 (CHEMBL1085780 | N-benzyl-2-(pyrrolidin-1-yl)pyrimi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342160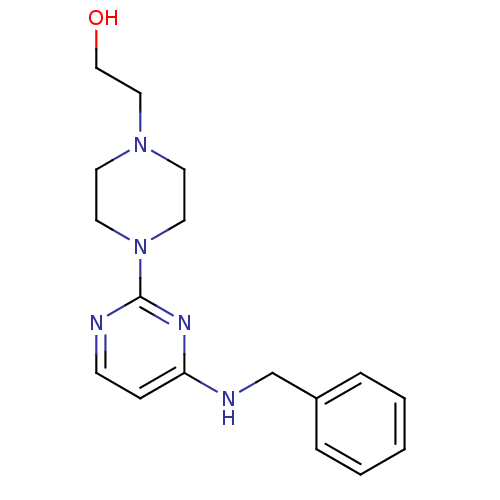 (2-[4-(4-(Benzylamino)pyrimidin-2-yl)piperazin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342159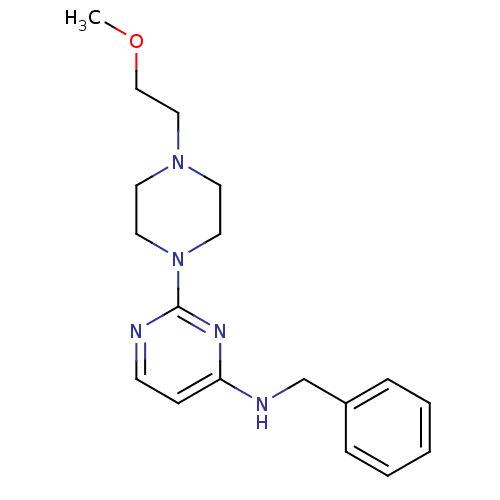 (CHEMBL1766141 | N-Benzyl-2-[4-(2-methoxyethyl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319979 (2-morpholino-N-(naphthalen-1-ylmethyl)pyrimidin-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50342155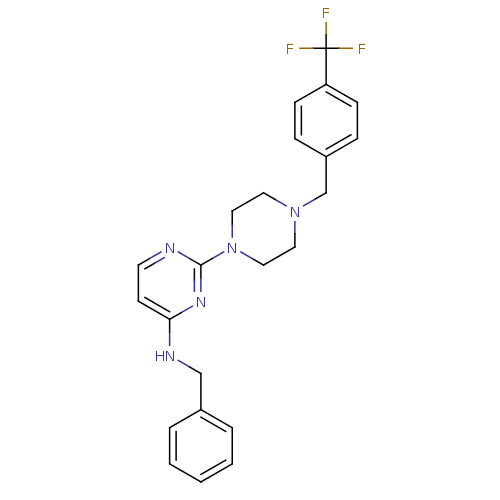 (CHEMBL1766145 | N-Benzyl-2-[4-(4-trifluoromethylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319980 (CHEMBL1084212 | N-(naphthalen-1-ylmethyl)-2-thiomo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342161 (CHEMBL1766139 | N-Benzyl-2-(4-propylpiperazin-1-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319969 (CHEMBL1085781 | N-benzyl-2-morpholinopyrimidin-4-a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319975 (CHEMBL1082979 | N-phenethyl-2-thiomorpholinopyrimi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319976 (2-(4-methylpiperazin-1-yl)-N-phenethylpyrimidin-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342150 (1-[4-(4-(Phenethylamino)pyrimidin-2-yl)piperazin-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342159 (CHEMBL1766141 | N-Benzyl-2-[4-(2-methoxyethyl)pipe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342151 (CHEMBL1766149 | N-benzyl-2-(piperazin-1-yl)pyrimid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319974 (2-morpholino-N-phenethylpyrimidin-4-amine | CHEMBL...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342155 (CHEMBL1766145 | N-Benzyl-2-[4-(4-trifluoromethylbe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342146 (CHEMBL1766017 | tert-Butyl 4-[4-(Benzylamino)pyrim...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50319971 (CHEMBL1084808 | N-benzyl-2-(4-methylpiperazin-1-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342160 (2-[4-(4-(Benzylamino)pyrimidin-2-yl)piperazin-1-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342147 (1-[4-(4-(Benzylamino)pyrimidin-2-yl)piperazin-1-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342153 (4-[4-(benzylamino)pyrimidin-2-yl]thiomorpholine-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50342152 (4-[4-(benzylamino)pyrimidin-2-yl]thiomorpholine-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||