

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343025 ((R)-4-(3-(methylamino)pyrrolidin-1-yl)-N2-neopenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343021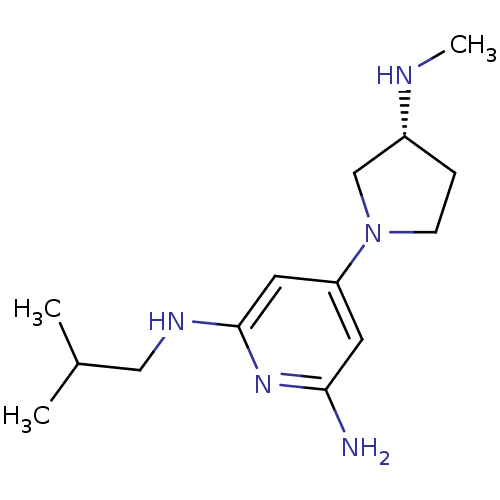 ((R)-N2-isobutyl-4-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343018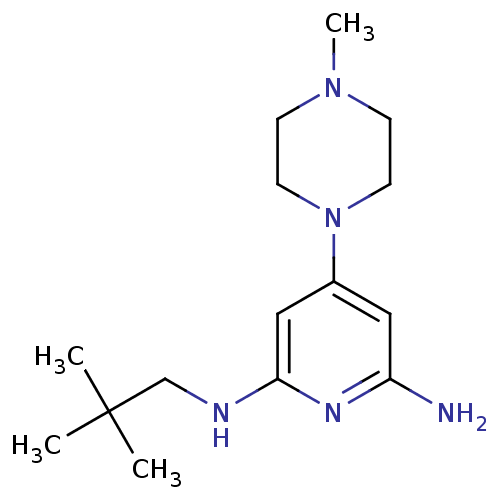 (4-(4-methylpiperazin-1-yl)-N2-neopentylpyridine-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343023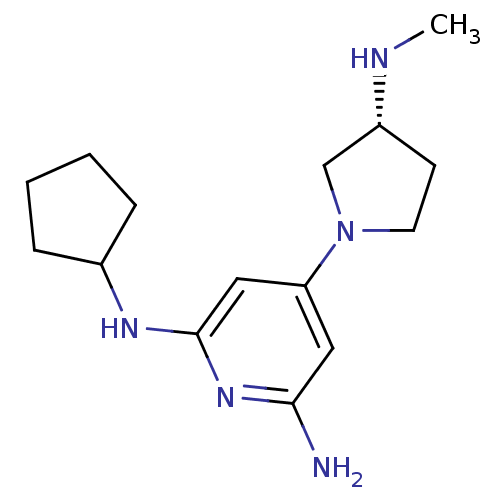 ((R)-N2-cyclopentyl-4-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343007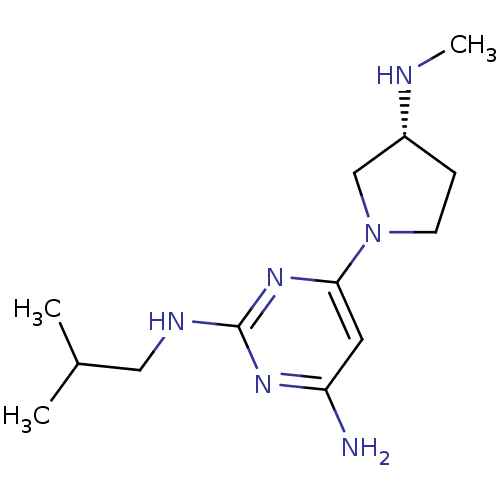 ((R)-N2-isobutyl-6-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342990 (CHEMBL1771001 | N4-cyclopentyl-6-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343014 (CHEMBL1770964 | N2-isobutyl-4-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342989 (CHEMBL1771000 | N4-isobutyl-6-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343019 ((R)-N2-isopropyl-4-(3-(methylamino)pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342987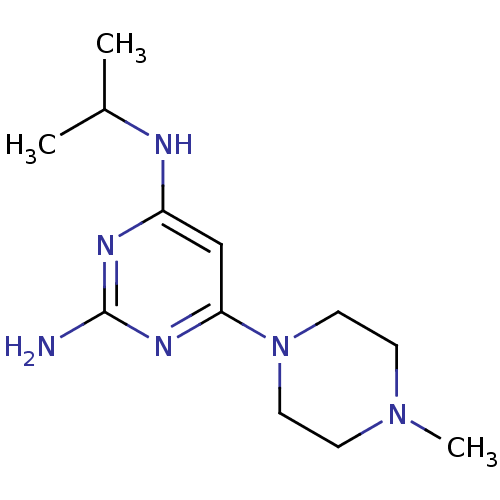 (CHEMBL1770998 | N4-isopropyl-6-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343016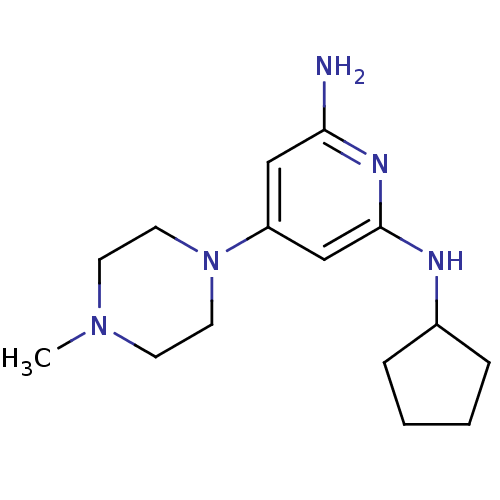 (CHEMBL1770966 | N2-cyclopentyl-4-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342992 ((R)-N4-isopropyl-6-(3-(methylamino)pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342994 ((R)-N4-isobutyl-6-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342982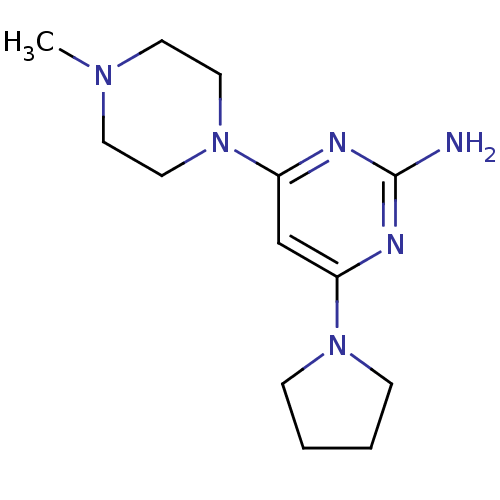 (4-(4-Methylpiperazin-1-yl)-6-pyrrolidin-1-ylpyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343009 ((R)-N2-cyclopentyl-6-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343005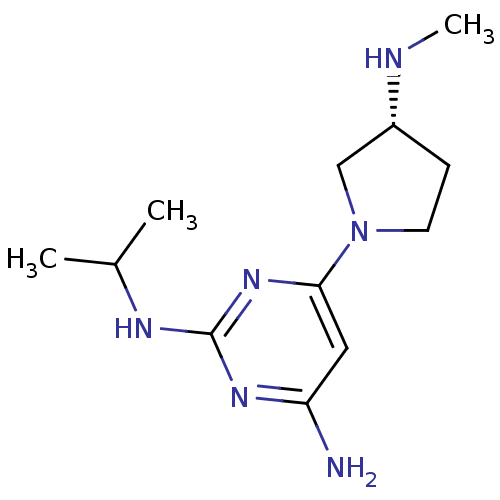 ((R)-N2-isopropyl-6-(3-(methylamino)pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342981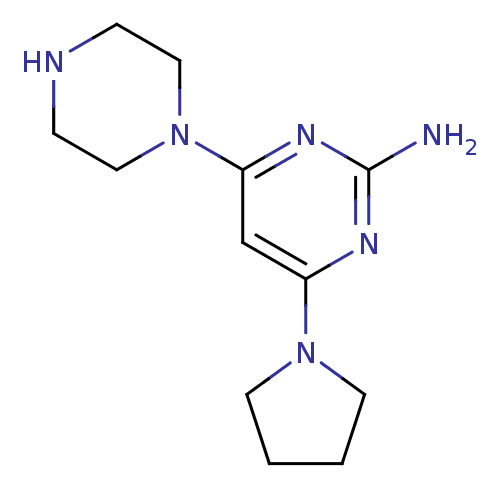 (4-(piperazin-1-yl)-6-(pyrrolidin-1-yl)pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342995 ((R)-N4-cyclopentyl-6-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343002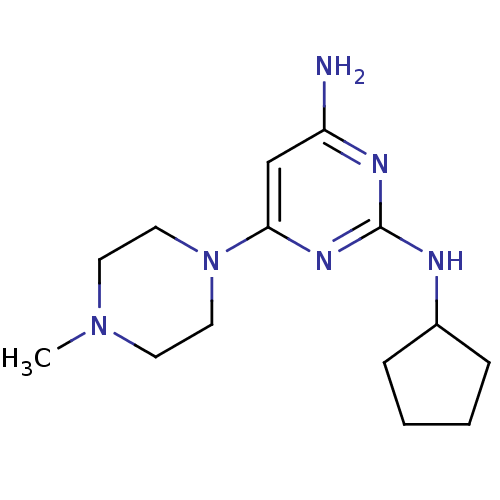 (CHEMBL1770987 | N2-cyclopentyl-6-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343000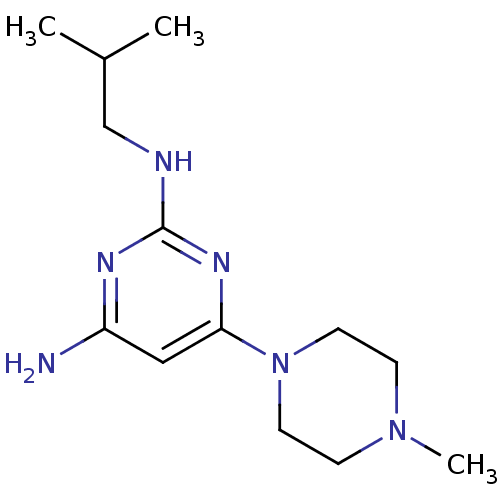 (CHEMBL1770985 | N2-isobutyl-6-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50304527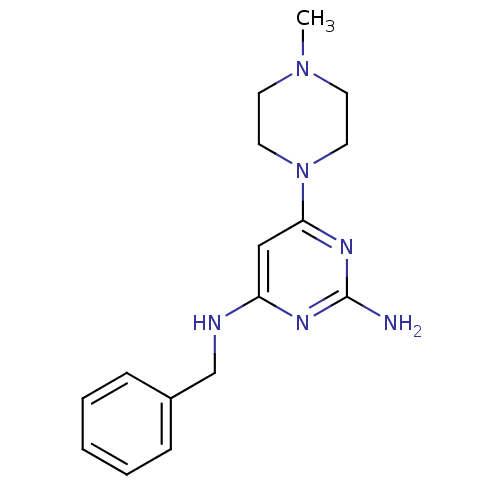 (CHEMBL492677 | N4-Benzyl-6-(4-methylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342988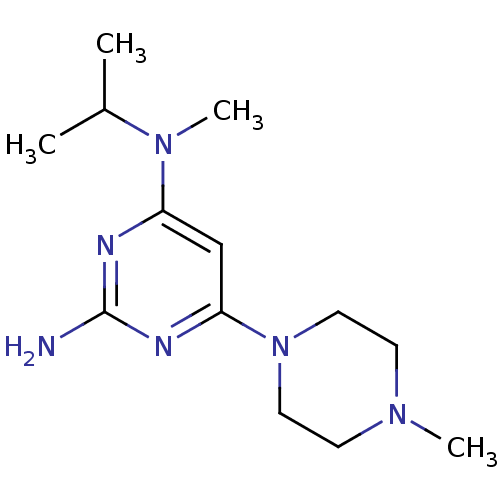 (CHEMBL1770999 | N4-isopropyl-N4-methyl-6-(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342991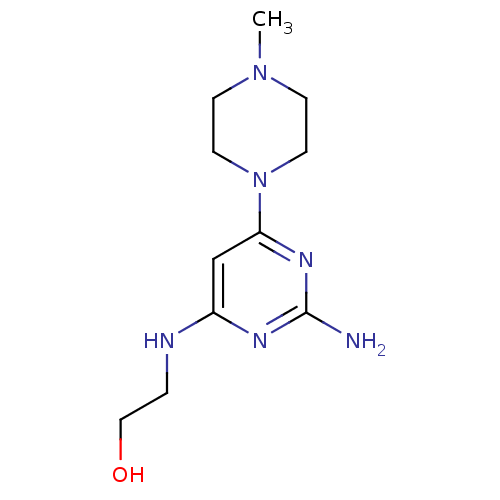 (2-(2-amino-6-(4-methylpiperazin-1-yl)pyrimidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335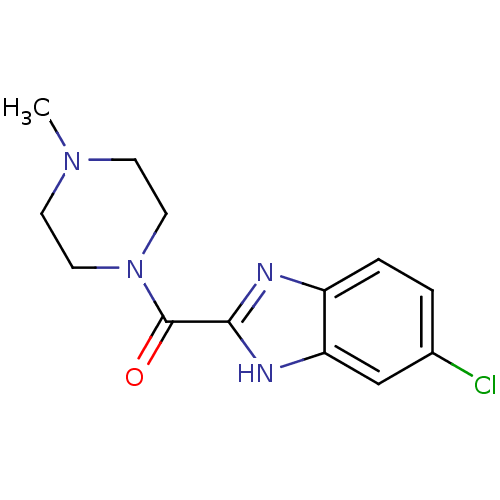 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343012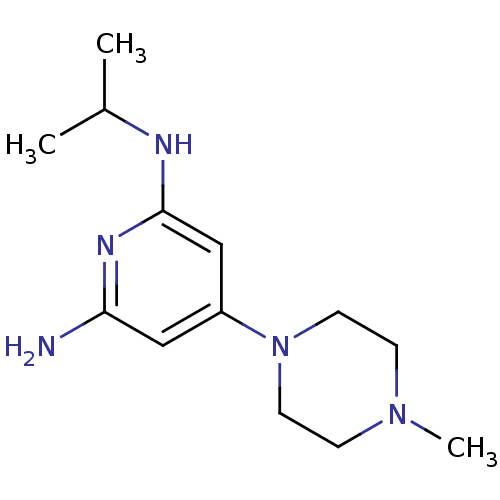 (CHEMBL1770962 | N2-isopropyl-4-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343020 ((R)-N2-isopropyl-N2-methyl-4-(3-(methylamino)pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342984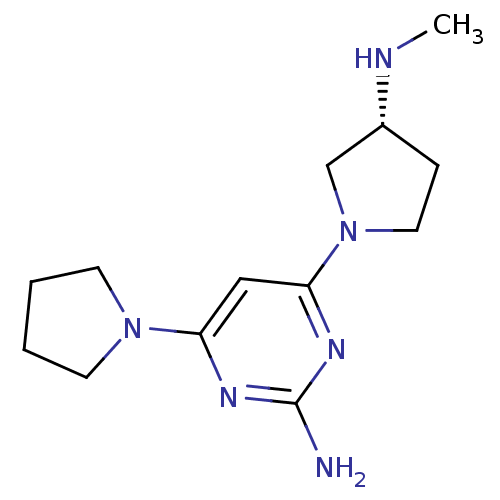 ((R)-4-(3-(methylamino)pyrrolidin-1-yl)-6-(pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342986 (CHEMBL1770997 | rac-4-(pyrrolidin-1-yl)-6-(tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342997 ((R)-2-(2-amino-6-(3-(methylamino)pyrrolidin-1-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343024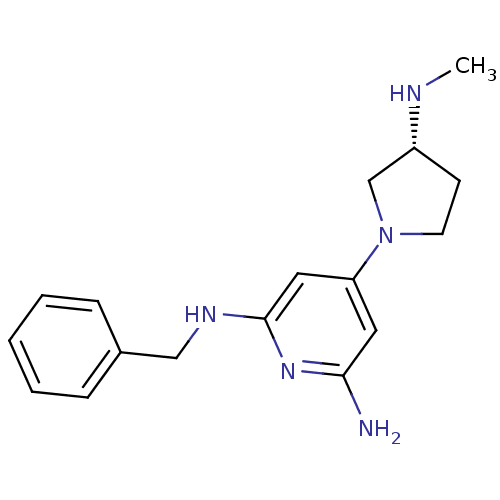 ((R)-N2-benzyl-4-(3-(methylamino)pyrrolidin-1-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342993 ((R)-N4-isopropyl-N4-methyl-6-(3-(methylamino)pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342996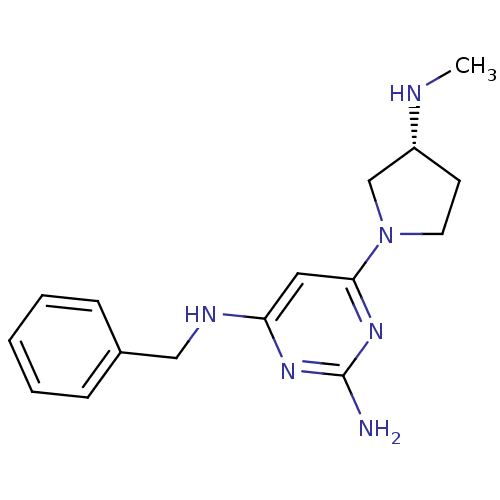 ((R)-N4-benzyl-6-(3-(methylamino)pyrrolidin-1-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342983 ((R)-4-(3-aminopyrrolidin-1-yl)-6-(pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343010 ((R)-N2-benzyl-6-(3-(methylamino)pyrrolidin-1-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343022 ((R)-4-(3-(methylamino)pyrrolidin-1-yl)-6-(pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342985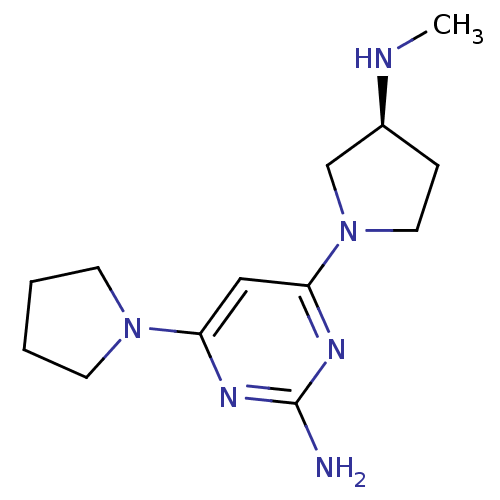 ((S)-4-(3-(methylamino)pyrrolidin-1-yl)-6-(pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343015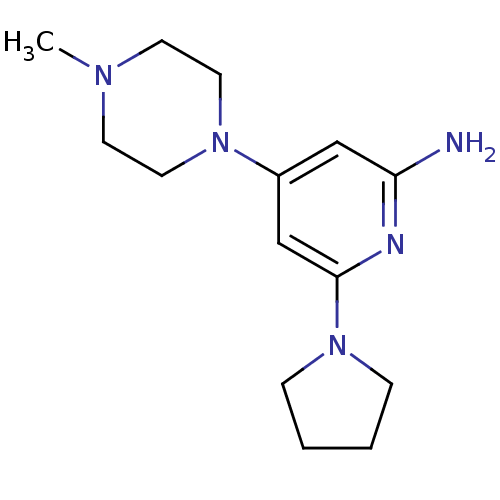 (4-(4-methylpiperazin-1-yl)-6-(pyrrolidin-1-yl)pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343008 ((R)-6-(3-(methylamino)pyrrolidin-1-yl)-2-(pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343001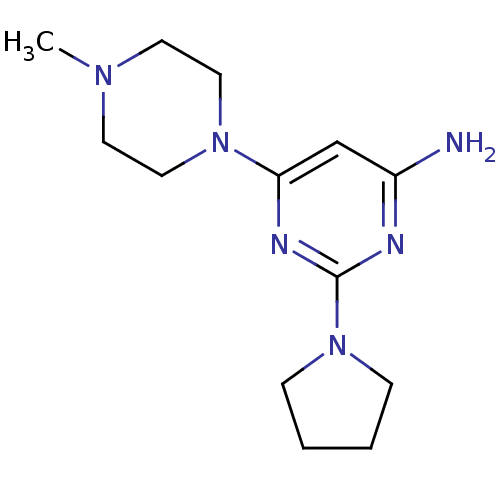 (6-(4-methylpiperazin-1-yl)-2-(pyrrolidin-1-yl)pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343006 ((R)-N2-isopropyl-N2-methyl-6-(3-(methylamino)pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343017 (CHEMBL1770967 | N2-benzyl-4-(4-methylpiperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342998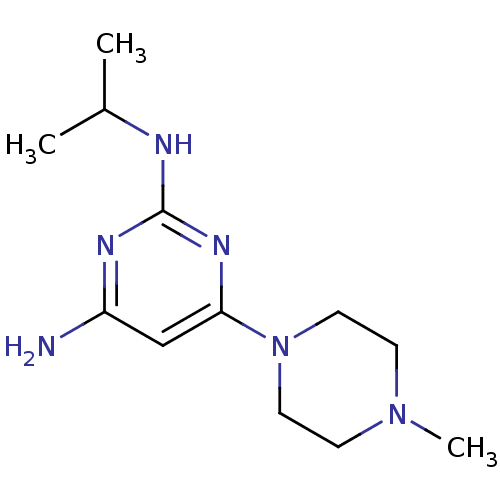 (CHEMBL1770983 | N2-isopropyl-6-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343013 (CHEMBL1770963 | N2-isopropyl-N2-methyl-4-(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343011 ((R)-2-(4-amino-6-(3-(methylamino)pyrrolidin-1-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342999 (CHEMBL1770984 | N2-isopropyl-N2-methyl-6-(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343003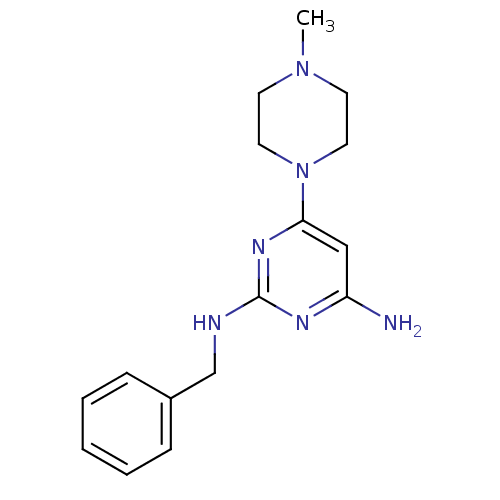 (CHEMBL1770988 | N2-benzyl-6-(4-methylpiperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343004 (2-(4-amino-6-(4-methylpiperazin-1-yl)pyrimidin-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343016 (CHEMBL1770966 | N2-cyclopentyl-4-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342987 (CHEMBL1770998 | N4-isopropyl-6-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342988 (CHEMBL1770999 | N4-isopropyl-N4-methyl-6-(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343000 (CHEMBL1770985 | N2-isobutyl-6-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343023 ((R)-N2-cyclopentyl-4-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342981 (4-(piperazin-1-yl)-6-(pyrrolidin-1-yl)pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342990 (CHEMBL1771001 | N4-cyclopentyl-6-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 101 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343021 ((R)-N2-isobutyl-4-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343015 (4-(4-methylpiperazin-1-yl)-6-(pyrrolidin-1-yl)pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342995 ((R)-N4-cyclopentyl-6-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342994 ((R)-N4-isobutyl-6-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342989 (CHEMBL1771000 | N4-isobutyl-6-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342982 (4-(4-Methylpiperazin-1-yl)-6-pyrrolidin-1-ylpyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343012 (CHEMBL1770962 | N2-isopropyl-4-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 533 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343019 ((R)-N2-isopropyl-4-(3-(methylamino)pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343009 ((R)-N2-cyclopentyl-6-(3-(methylamino)pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343005 ((R)-N2-isopropyl-6-(3-(methylamino)pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343022 ((R)-4-(3-(methylamino)pyrrolidin-1-yl)-6-(pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343014 (CHEMBL1770964 | N2-isobutyl-4-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343002 (CHEMBL1770987 | N2-cyclopentyl-6-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342984 ((R)-4-(3-(methylamino)pyrrolidin-1-yl)-6-(pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343007 ((R)-N2-isobutyl-6-(3-(methylamino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342992 ((R)-N4-isopropyl-6-(3-(methylamino)pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||