

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346448 (2-(2-(((2S,6S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346455 (2-(2-(((2S,5S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331048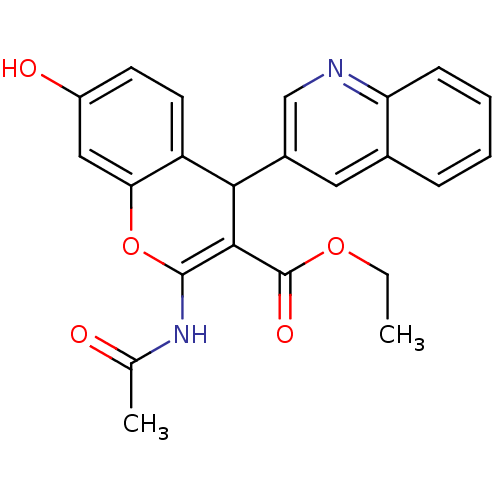 (CHEMBL1277437 | ethyl 2-acetamido-7-hydroxy-4-(qui...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346461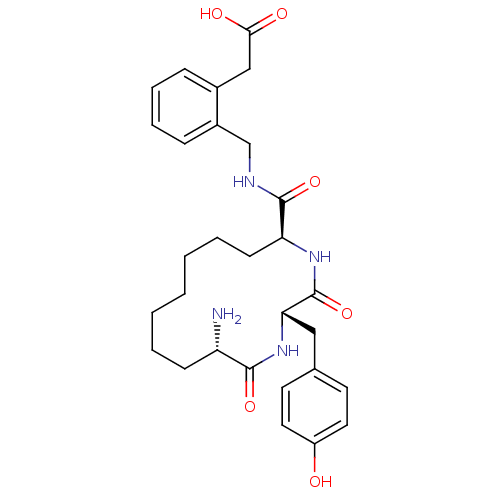 (2-(2-(((2S,5S,13S)-13-amino-2-(4-hydroxybenzyl)-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346452 (2-(2-(((2S,6S,13S,Z)-13-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346456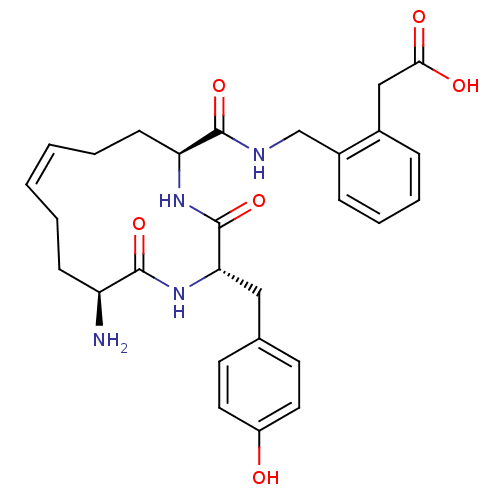 (2-(2-(((2S,5S,12S,Z)-12-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346451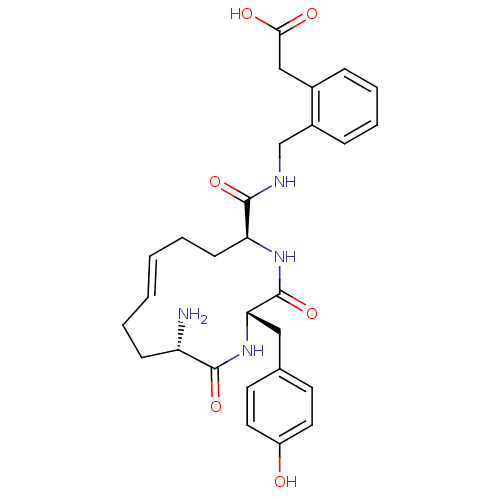 (2-(2-(((2S,5S,12S,E)-12-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346453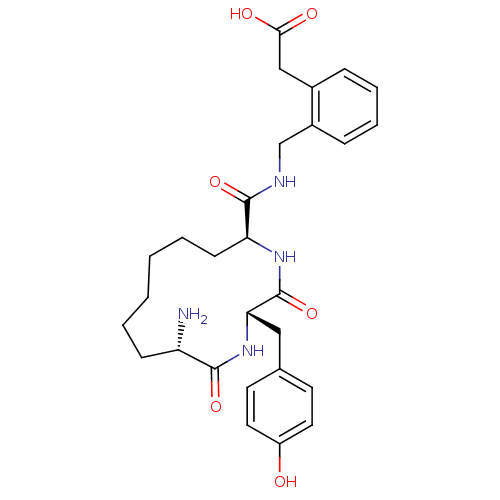 (2-(2-(((2S,5S,12S)-12-amino-2-(4-hydroxybenzyl)-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346458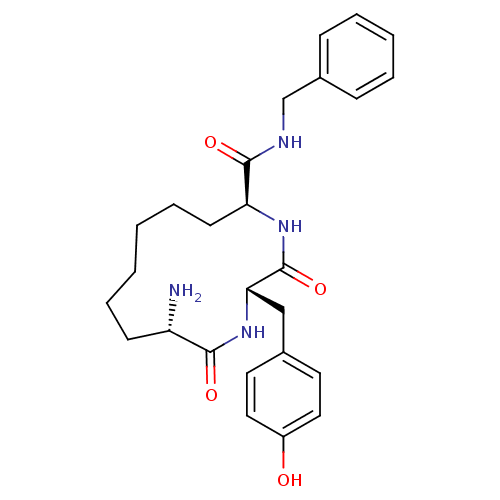 ((2S,5S,12S)-12-amino-N-benzyl-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346448 (2-(2-(((2S,6S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346449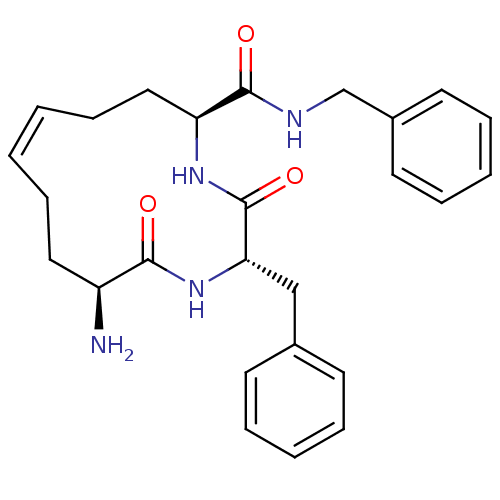 ((2S,5S,12S,Z)-12-amino-N,2-dibenzyl-3,13-dioxo-1,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346450 ((2S,5S,12S,E)-12-amino-N,2-dibenzyl-3,13-dioxo-1,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 697 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 841 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346451 (2-(2-(((2S,5S,12S,E)-12-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346452 (2-(2-(((2S,6S,13S,Z)-13-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346450 ((2S,5S,12S,E)-12-amino-N,2-dibenzyl-3,13-dioxo-1,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346453 (2-(2-(((2S,5S,12S)-12-amino-2-(4-hydroxybenzyl)-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346454 ((2S,5S,13S,Z)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346455 (2-(2-(((2S,5S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346456 (2-(2-(((2S,5S,12S,Z)-12-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346457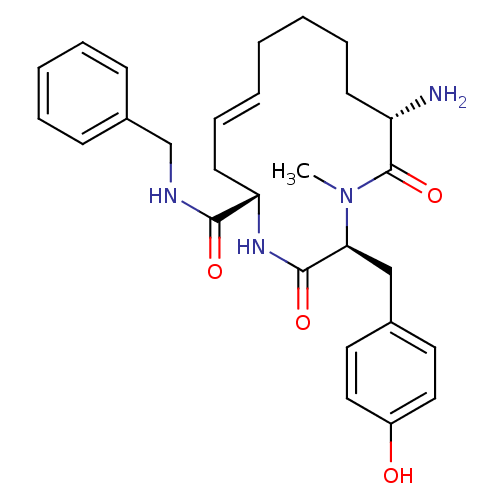 ((2S,5S,13S,E)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346458 ((2S,5S,12S)-12-amino-N-benzyl-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346459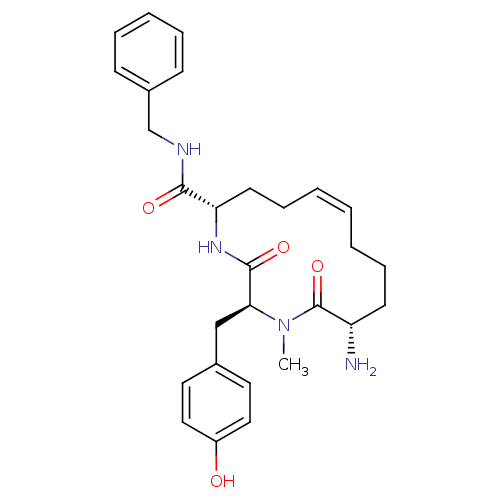 ((2S,5S,13S,Z)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346449 ((2S,5S,12S,Z)-12-amino-N,2-dibenzyl-3,13-dioxo-1,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346461 (2-(2-(((2S,5S,13S)-13-amino-2-(4-hydroxybenzyl)-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346460 ((2S,5S,13S,E)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346460 ((2S,5S,13S,E)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346459 ((2S,5S,13S,Z)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346454 ((2S,5S,13S,Z)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50346457 ((2S,5S,13S,E)-13-amino-N-benzyl-2-(4-hydroxybenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human AP-N transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||