

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343131 (2-(2-cyanopyrimidin-5-yl)-N-(3-ethylphenyl)-4-o-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343129 (2-(4-cyanopyridin-3-yl)-N-(3-ethylphenyl)-4-o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343132 (2-(3,5-dimethylisoxazol-4-yl)-N-(3-ethylphenyl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343120 (4-(2,6-dimethylphenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343128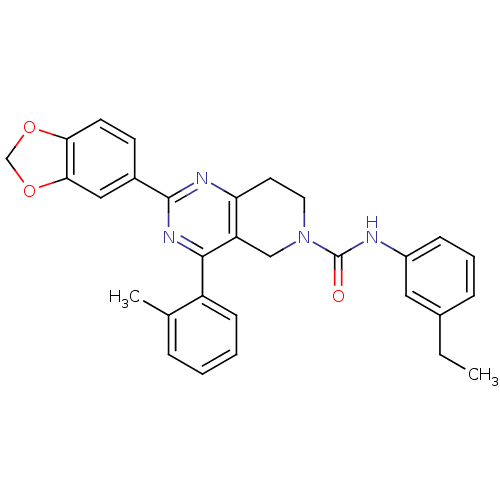 (2-(benzo[d][1,3]dioxol-5-yl)-N-(3-ethylphenyl)-4-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343130 (CHEMBL1771458 | N-(3-ethylphenyl)-2-(pyrimidin-5-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343127 (CHEMBL1771455 | N-(3-ethylphenyl)-2-(quinolin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343094 (2-(4-cyanophenyl)-N-(3-ethylphenyl)-4-o-tolyl-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343095 (CHEMBL1771452 | N-(3-ethylphenyl)-2-(4-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343109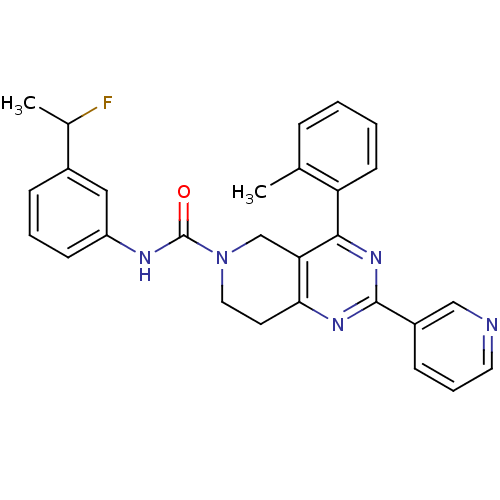 (CHEMBL1771244 | N-(3-(1-fluoroethyl)phenyl)-2-(pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343125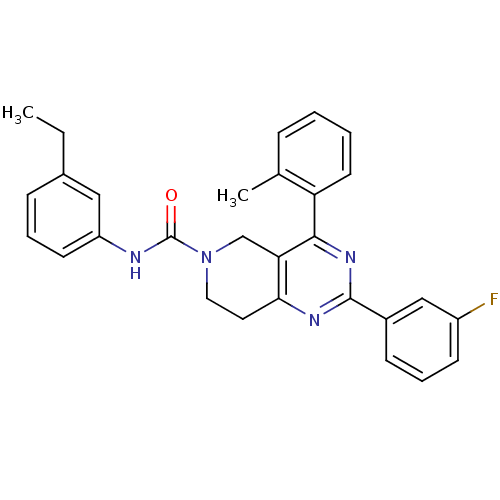 (CHEMBL1771453 | N-(3-ethylphenyl)-2-(3-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343121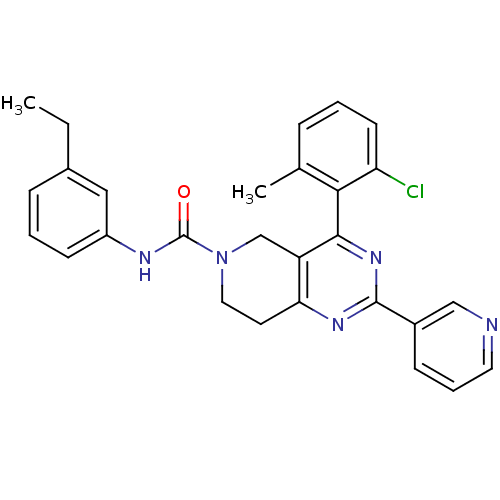 (4-(2-chloro-6-methylphenyl)-N-(3-ethylphenyl)-2-(p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343122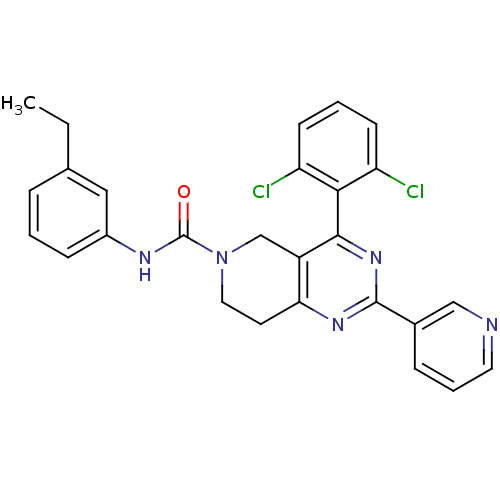 (4-(2,6-dichlorophenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343090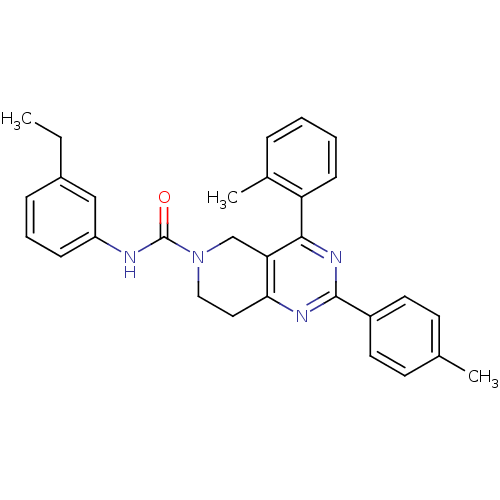 (CHEMBL1771446 | N-(3-ethylphenyl)-4-o-tolyl-2-p-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343103 (CHEMBL1771238 | N-(3-ethylphenyl)-2-(pyridin-3-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343089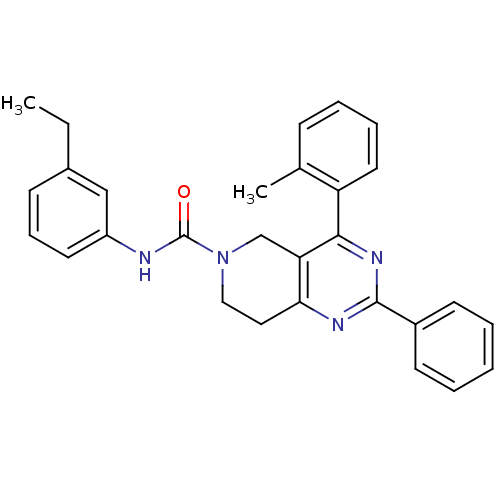 (CHEMBL1771445 | N-(3-ethylphenyl)-2-phenyl-4-o-tol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343093 (2-(3-cyanophenyl)-N-(3-ethylphenyl)-4-o-tolyl-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343104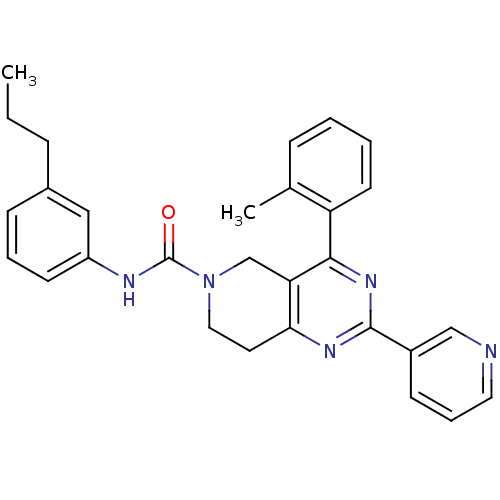 (CHEMBL1771239 | N-(3-propylphenyl)-2-(pyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343111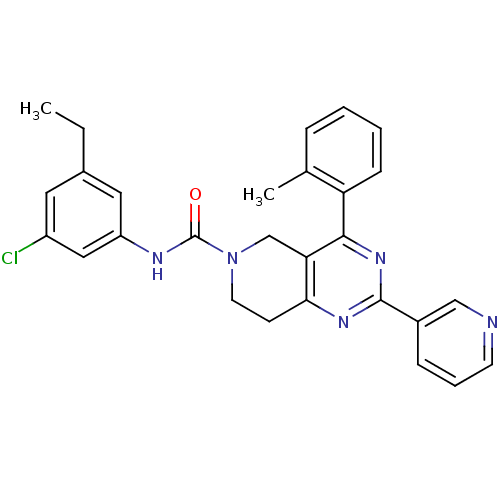 (CHEMBL1771246 | N-(3-chloro-5-ethylphenyl)-2-(pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343116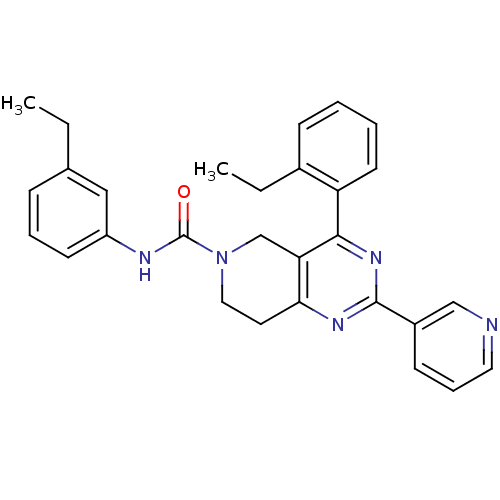 (4-(2-ethylphenyl)-N-(3-ethylphenyl)-2-(pyridin-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343100 (CHEMBL1771235 | N-(3-ethoxyphenyl)-2-(pyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343112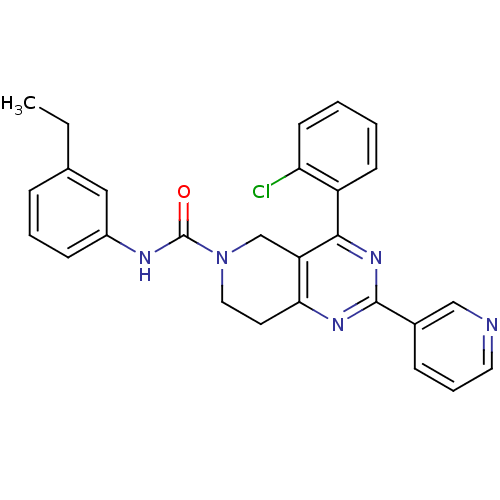 (4-(2-chlorophenyl)-N-(3-ethylphenyl)-2-(pyridin-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343097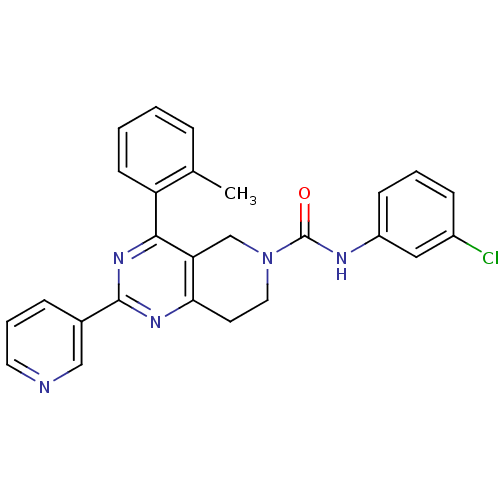 (CHEMBL1771232 | N-(3-chlorophenyl)-2-(pyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343110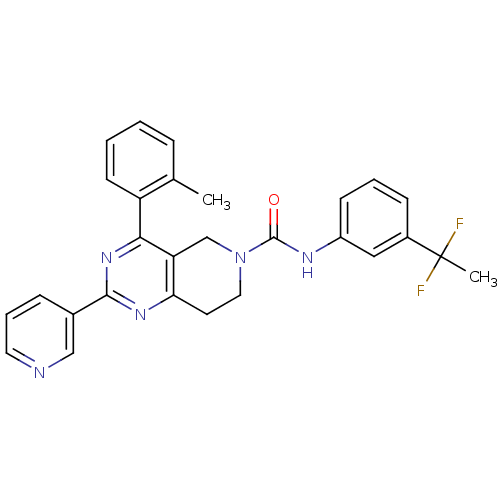 (CHEMBL1771245 | N-(3-(1,1-difluoroethyl)phenyl)-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343101 (CHEMBL1771236 | N-(3-(methylthio)phenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343126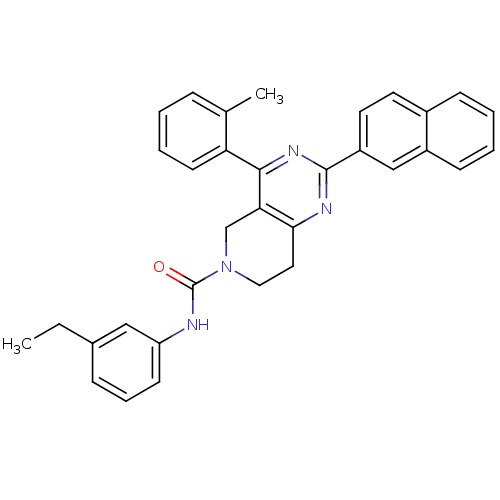 (CHEMBL1771454 | N-(3-ethylphenyl)-2-(naphthalen-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343119 (CHEMBL1771438 | N-(3-ethylphenyl)-4-(2-(hydroxymet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343106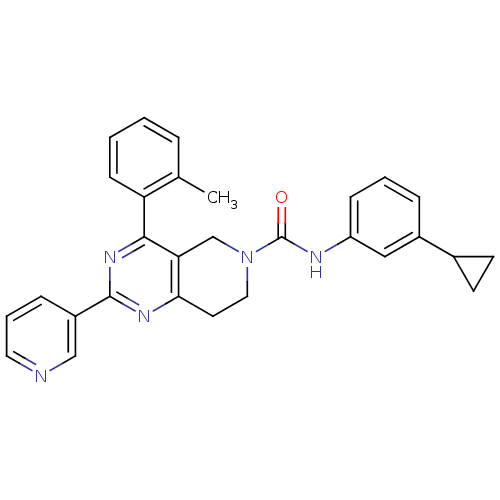 (CHEMBL1771241 | N-(3-cyclopropylphenyl)-2-(pyridin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343105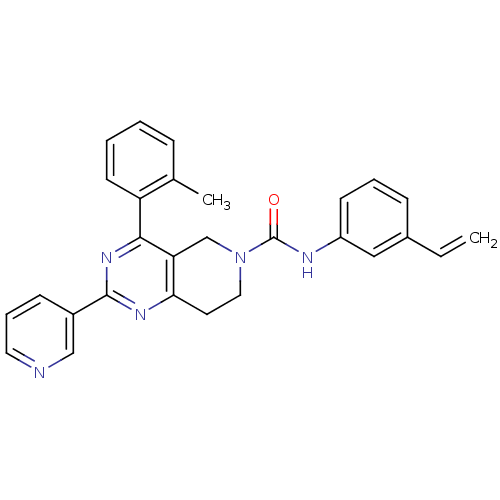 (2-(pyridin-3-yl)-4-o-tolyl-N-(3-vinylphenyl)-7,8-d...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343091 (CHEMBL1771447 | N-(3-ethylphenyl)-2-m-tolyl-4-o-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343124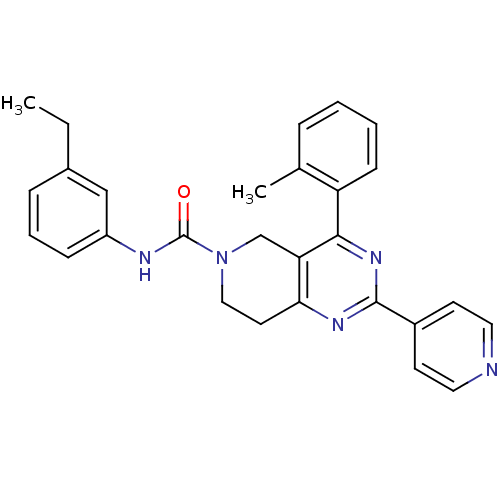 (CHEMBL1771444 | N-(3-ethylphenyl)-2-(pyridin-4-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343102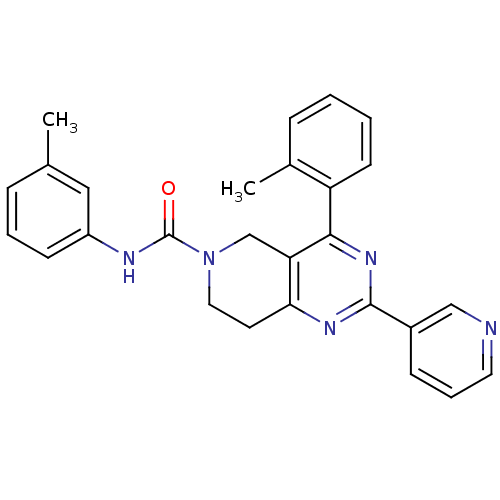 (2-(pyridin-3-yl)-N-m-tolyl-4-o-tolyl-7,8-dihydropy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343123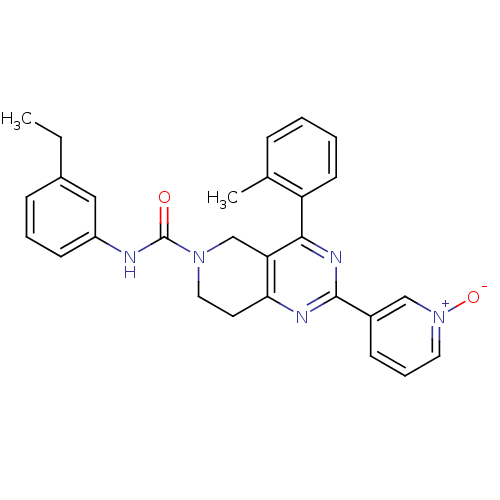 (3-(6-(3-ethylphenylcarbamoyl)-4-o-tolyl-5,6,7,8-te...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343117 (CHEMBL1771436 | N-(3-ethylphenyl)-4-(2-methoxyphen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343113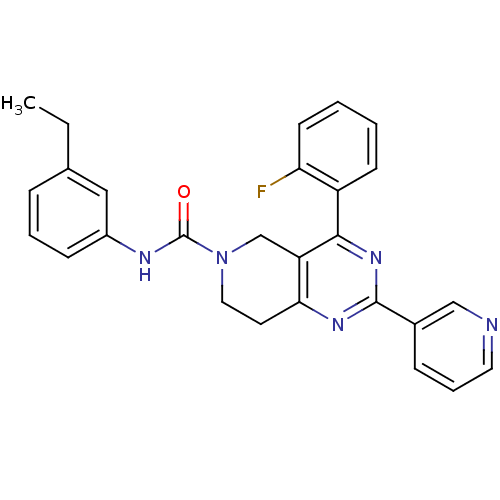 (CHEMBL1771248 | N-(3-ethylphenyl)-4-(2-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343096 (CHEMBL1771230 | N-phenyl-2-(pyridin-3-yl)-4-o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50343132 (2-(3,5-dimethylisoxazol-4-yl)-N-(3-ethylphenyl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [35S]MK-0499 from human ERG | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50343128 (2-(benzo[d][1,3]dioxol-5-yl)-N-(3-ethylphenyl)-4-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [35S]MK-0499 from human ERG | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50343130 (CHEMBL1771458 | N-(3-ethylphenyl)-2-(pyrimidin-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [35S]MK-0499 from human ERG | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343099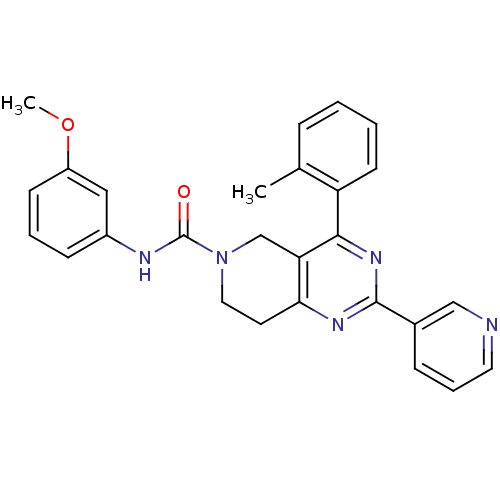 (CHEMBL1771234 | N-(3-methoxyphenyl)-2-(pyridin-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343098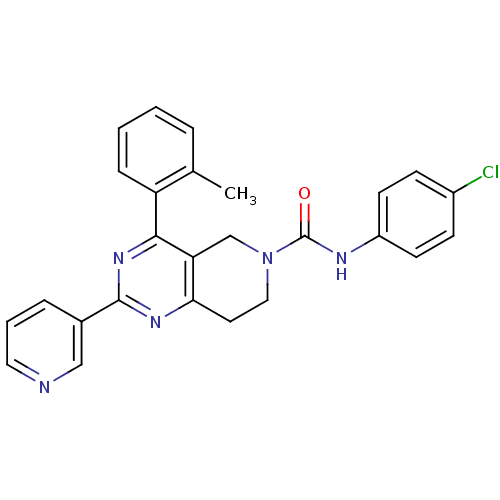 (CHEMBL1771233 | N-(4-chlorophenyl)-2-(pyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343118 (CHEMBL1771437 | N-(3-ethylphenyl)-4-(2-hydroxyphen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343107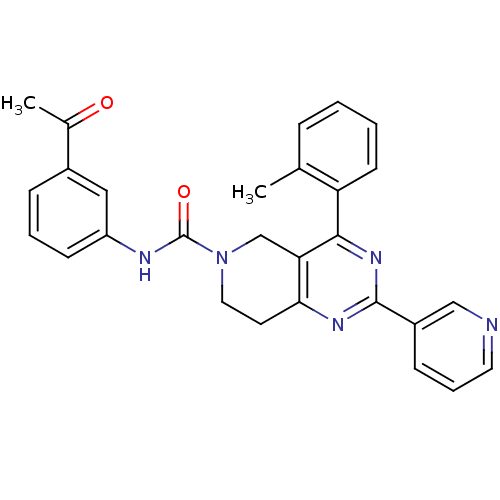 (CHEMBL1771242 | N-(3-acetylphenyl)-2-(pyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343115 (4-(4-chloro-2-methylphenyl)-N-(3-ethylphenyl)-2-(p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343092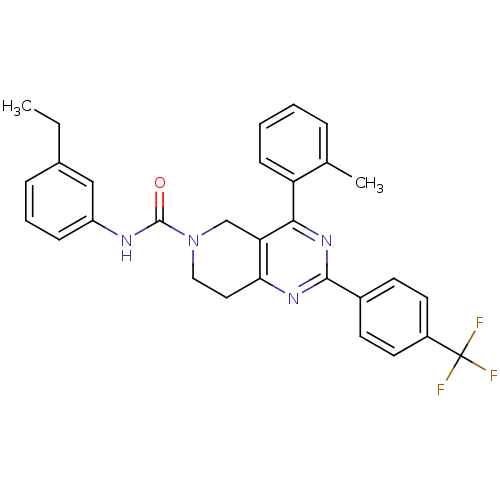 (CHEMBL1771449 | N-(3-ethylphenyl)-4-o-tolyl-2-(4-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343108 (CHEMBL1771243 | N-(3-(1-hydroxyethyl)phenyl)-2-(py...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343114 (4-(3-chlorophenyl)-N-(3-ethylphenyl)-2-(pyridin-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||