 Found 69 hits of Enzyme Inhibition Constant Data
Found 69 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50032651
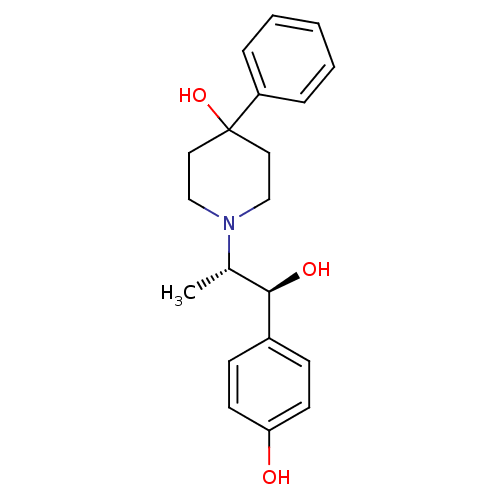
(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344250
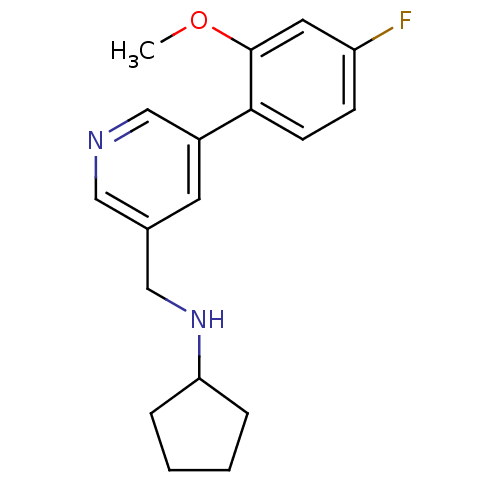
(CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-9-15(19)6-7-17(18)14-8-13(10-20-12-14)11-21-16-4-2-3-5-16/h6-10,12,16,21H,2-5,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344232
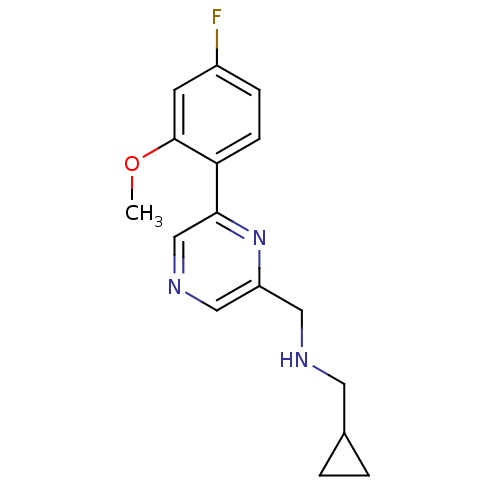
(1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...)Show InChI InChI=1S/C16H18FN3O/c1-21-16-6-12(17)4-5-14(16)15-10-19-9-13(20-15)8-18-7-11-2-3-11/h4-6,9-11,18H,2-3,7-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344251
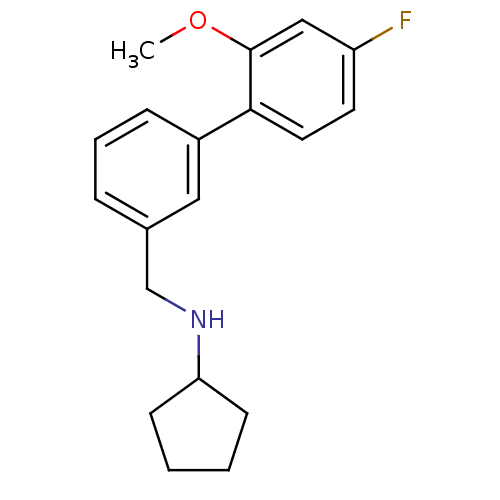
(CHEMBL1779004 | N-((4'-fluoro-2'-methoxybiphenyl-3...)Show InChI InChI=1S/C19H22FNO/c1-22-19-12-16(20)9-10-18(19)15-6-4-5-14(11-15)13-21-17-7-2-3-8-17/h4-6,9-12,17,21H,2-3,7-8,13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344233
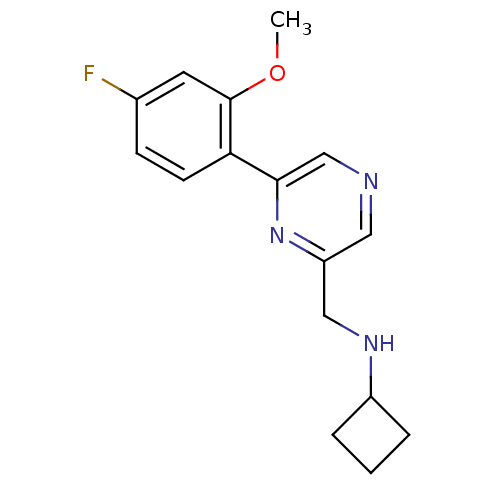
(CHEMBL1778866 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C16H18FN3O/c1-21-16-7-11(17)5-6-14(16)15-10-18-8-13(20-15)9-19-12-3-2-4-12/h5-8,10,12,19H,2-4,9H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344234
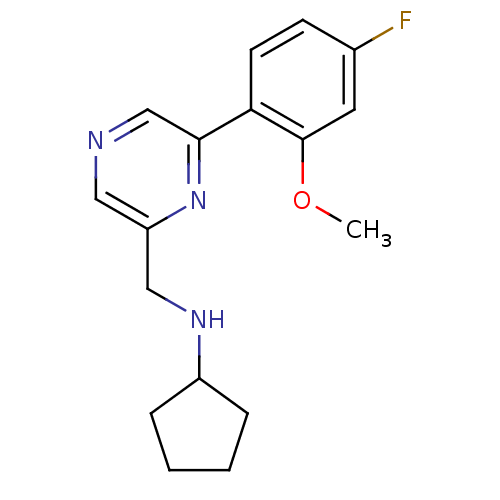
(CHEMBL1778867 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C17H20FN3O/c1-22-17-8-12(18)6-7-15(17)16-11-19-9-14(21-16)10-20-13-4-2-3-5-13/h6-9,11,13,20H,2-5,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344252
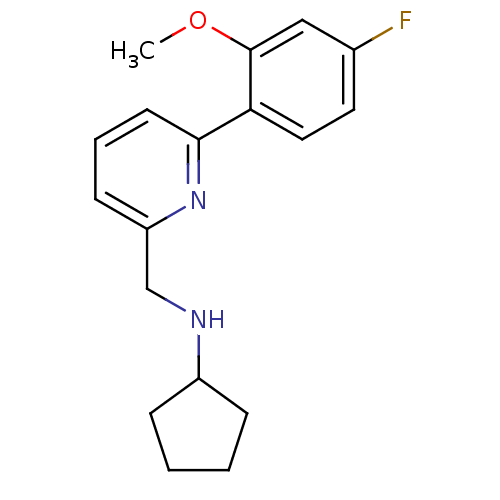
(CHEMBL1779005 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-11-13(19)9-10-16(18)17-8-4-7-15(21-17)12-20-14-5-2-3-6-14/h4,7-11,14,20H,2-3,5-6,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344259

(CHEMBL1779012 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H15FN4O2/c1-25-17-7-14(19)2-3-15(17)13-6-12(8-21-10-13)9-23-18(24)16-11-20-4-5-22-16/h2-8,10-11H,9H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344254

(CHEMBL1779007 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H23FN2O/c1-23-19-10-16(20)7-8-18(19)15-9-14(11-21-13-15)12-22-17-5-3-2-4-6-17/h7-11,13,17,22H,2-6,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344260
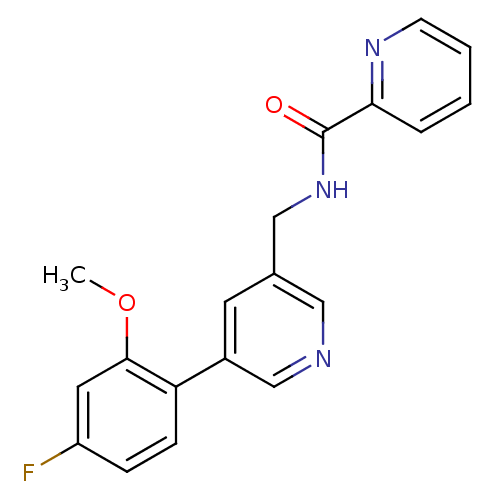
(CHEMBL1779013 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H16FN3O2/c1-25-18-9-15(20)5-6-16(18)14-8-13(10-21-12-14)11-23-19(24)17-4-2-3-7-22-17/h2-10,12H,11H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344241
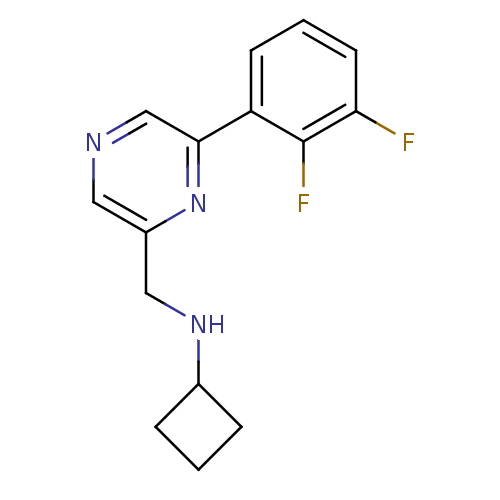
(CHEMBL1778994 | N-((6-(2,3-difluorophenyl)pyrazin-...)Show InChI InChI=1S/C15H15F2N3/c16-13-6-2-5-12(15(13)17)14-9-18-7-11(20-14)8-19-10-3-1-4-10/h2,5-7,9-10,19H,1,3-4,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344235
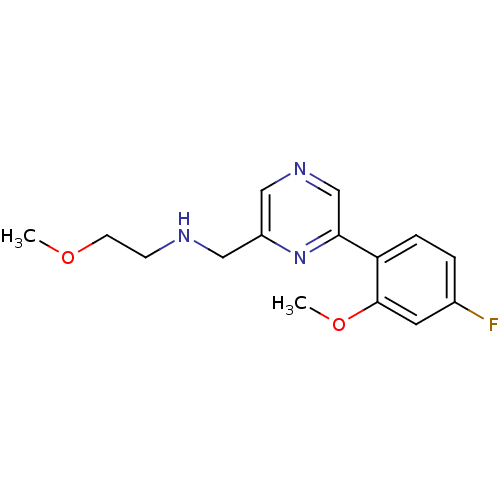
(CHEMBL1778868 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C15H18FN3O2/c1-20-6-5-17-8-12-9-18-10-14(19-12)13-4-3-11(16)7-15(13)21-2/h3-4,7,9-10,17H,5-6,8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344236
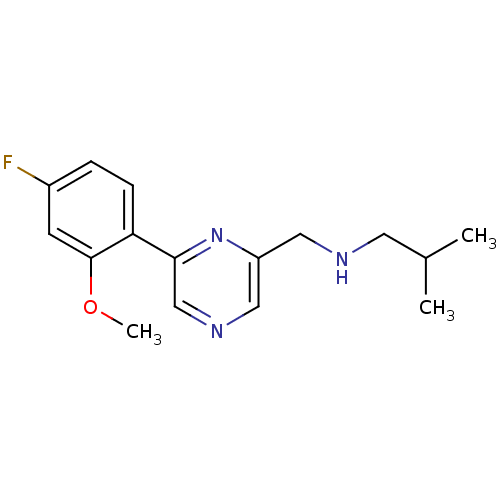
(CHEMBL1778869 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C16H20FN3O/c1-11(2)7-18-8-13-9-19-10-15(20-13)14-5-4-12(17)6-16(14)21-3/h4-6,9-11,18H,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344237
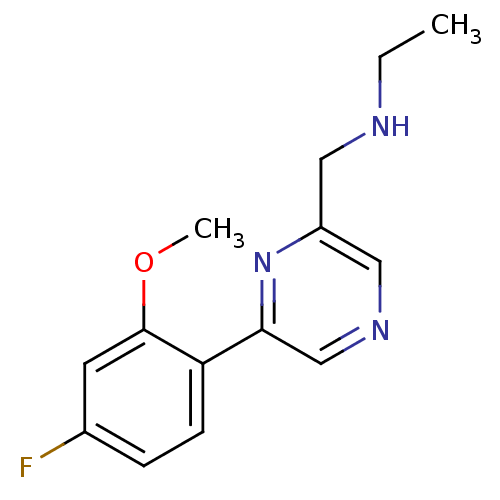
(CHEMBL1778870 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C14H16FN3O/c1-3-16-7-11-8-17-9-13(18-11)12-5-4-10(15)6-14(12)19-2/h4-6,8-9,16H,3,7H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344255
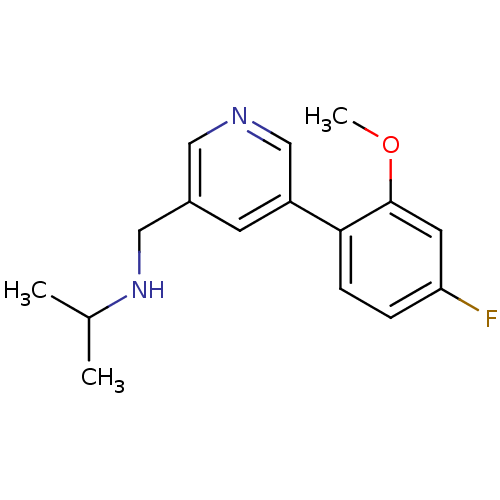
(CHEMBL1779008 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C16H19FN2O/c1-11(2)19-9-12-6-13(10-18-8-12)15-5-4-14(17)7-16(15)20-3/h4-8,10-11,19H,9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344262
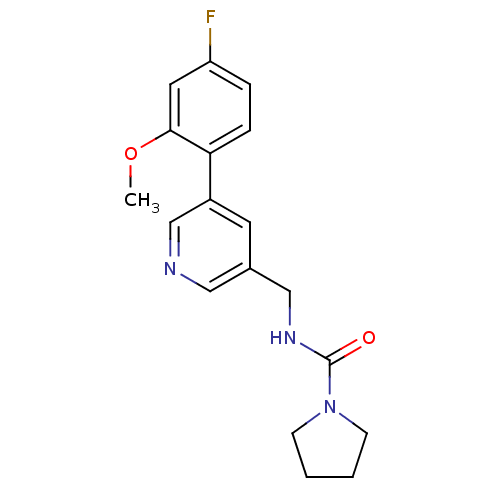
(CHEMBL1779015 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H20FN3O2/c1-24-17-9-15(19)4-5-16(17)14-8-13(10-20-12-14)11-21-18(23)22-6-2-3-7-22/h4-5,8-10,12H,2-3,6-7,11H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344264

(CHEMBL1779016 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H19FN2O2/c1-23-17-8-15(19)5-6-16(17)14-7-12(9-20-11-14)10-21-18(22)13-3-2-4-13/h5-9,11,13H,2-4,10H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344242

(1-cyclopropyl-N-((6-(2,3-difluorophenyl)pyrazin-2-...)Show InChI InChI=1S/C15H15F2N3/c16-13-3-1-2-12(15(13)17)14-9-19-8-11(20-14)7-18-6-10-4-5-10/h1-3,8-10,18H,4-7H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344265
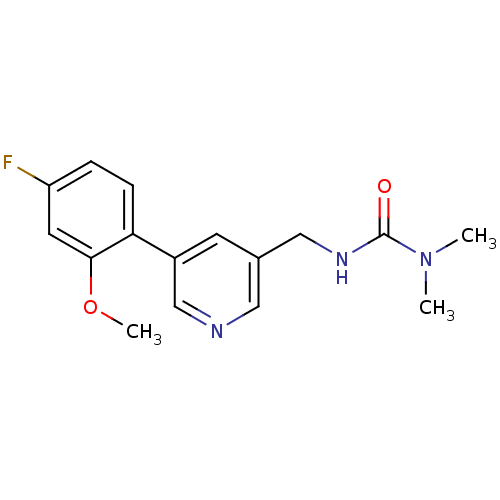
(3-((5-(4-fluoro-2-methoxyphenyl)pyridin-3-yl)methy...)Show InChI InChI=1S/C16H18FN3O2/c1-20(2)16(21)19-9-11-6-12(10-18-8-11)14-5-4-13(17)7-15(14)22-3/h4-8,10H,9H2,1-3H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344238
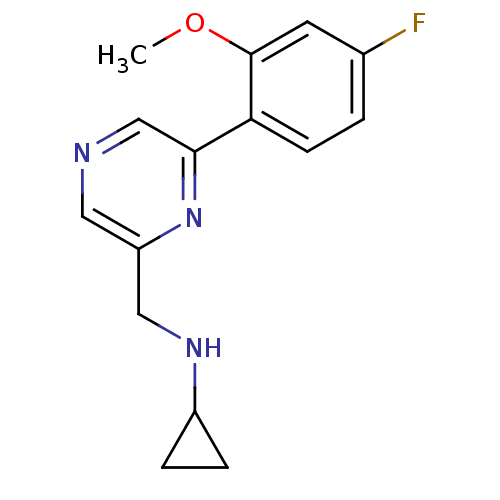
(CHEMBL1778991 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C15H16FN3O/c1-20-15-6-10(16)2-5-13(15)14-9-17-7-12(19-14)8-18-11-3-4-11/h2,5-7,9,11,18H,3-4,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 429 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344256
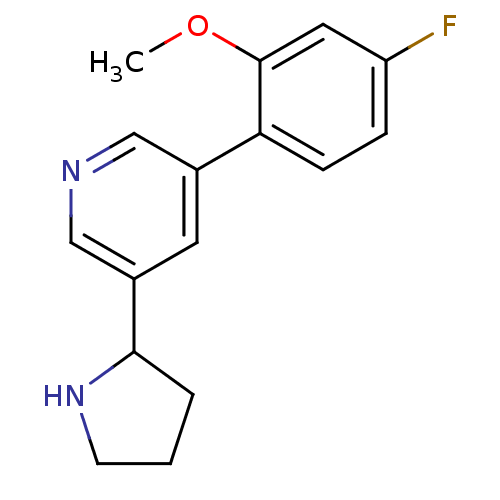
(3-(4-fluoro-2-methoxyphenyl)-5-(pyrrolidin-2-yl)py...)Show InChI InChI=1S/C16H17FN2O/c1-20-16-8-13(17)4-5-14(16)11-7-12(10-18-9-11)15-3-2-6-19-15/h4-5,7-10,15,19H,2-3,6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344261
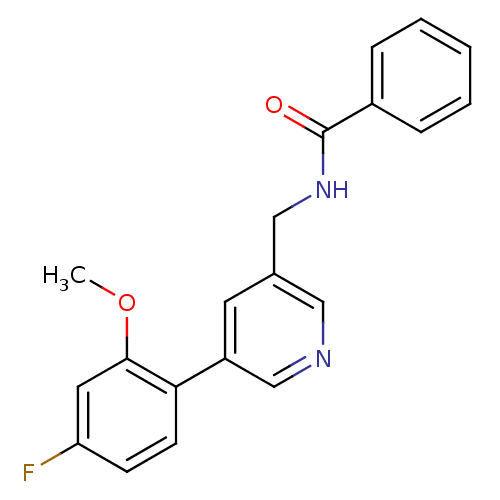
(CHEMBL1779014 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C20H17FN2O2/c1-25-19-10-17(21)7-8-18(19)16-9-14(11-22-13-16)12-23-20(24)15-5-3-2-4-6-15/h2-11,13H,12H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344257
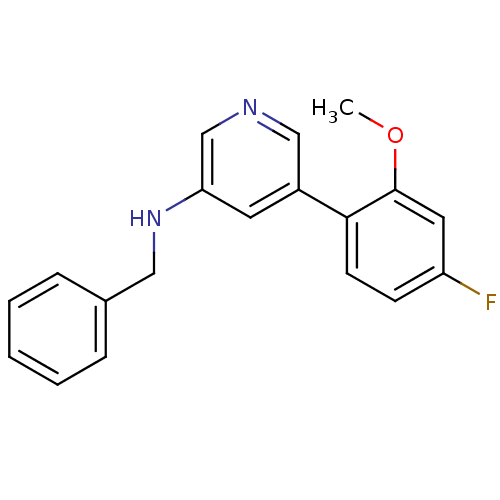
(CHEMBL1779010 | N-benzyl-5-(4-fluoro-2-methoxyphen...)Show InChI InChI=1S/C19H17FN2O/c1-23-19-10-16(20)7-8-18(19)15-9-17(13-21-12-15)22-11-14-5-3-2-4-6-14/h2-10,12-13,22H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344266
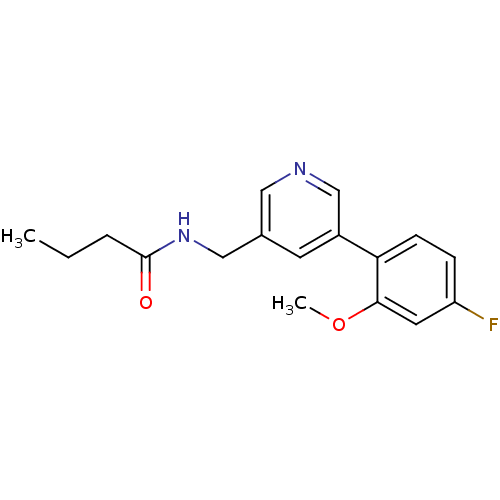
(CHEMBL1779018 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C17H19FN2O2/c1-3-4-17(21)20-10-12-7-13(11-19-9-12)15-6-5-14(18)8-16(15)22-2/h5-9,11H,3-4,10H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344267
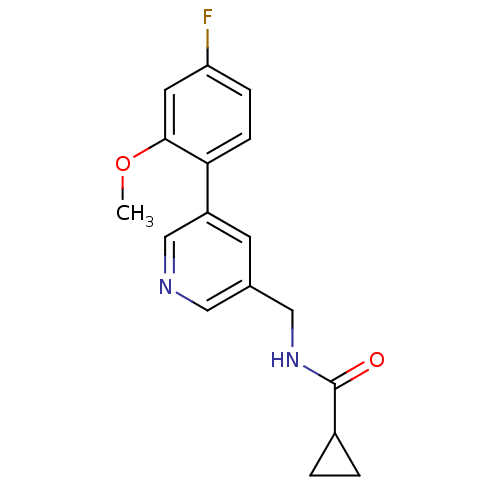
(CHEMBL1779019 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C17H17FN2O2/c1-22-16-7-14(18)4-5-15(16)13-6-11(8-19-10-13)9-20-17(21)12-2-3-12/h4-8,10,12H,2-3,9H2,1H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344244
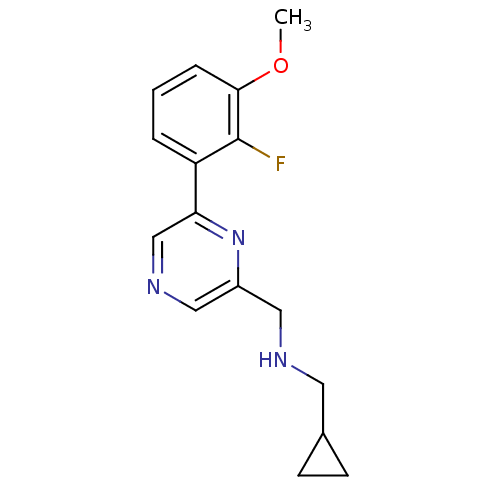
(1-cyclopropyl-N-((6-(2-fluoro-3-methoxyphenyl)pyra...)Show InChI InChI=1S/C16H18FN3O/c1-21-15-4-2-3-13(16(15)17)14-10-19-9-12(20-14)8-18-7-11-5-6-11/h2-4,9-11,18H,5-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344239
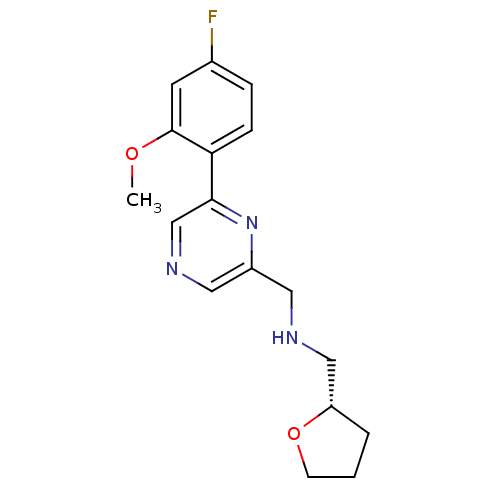
((S)-1-(6-(4-fluoro-2-methoxyphenyl)pyrazin-2-yl)-N...)Show SMILES COc1cc(F)ccc1-c1cncc(CNC[C@@H]2CCCO2)n1 |r| Show InChI InChI=1S/C17H20FN3O2/c1-22-17-7-12(18)4-5-15(17)16-11-20-9-13(21-16)8-19-10-14-3-2-6-23-14/h4-5,7,9,11,14,19H,2-3,6,8,10H2,1H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344258
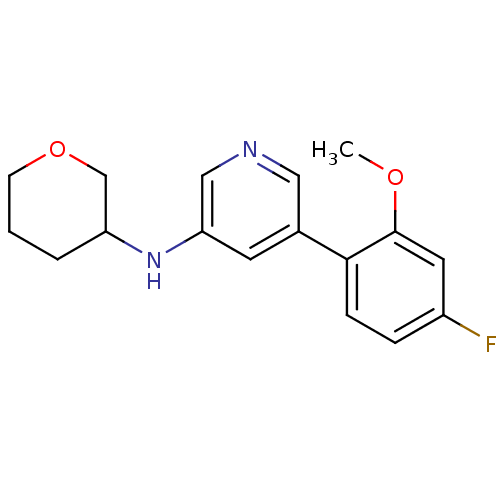
(5-(4-fluoro-2-methoxyphenyl)-N-(tetrahydro-2H-pyra...)Show InChI InChI=1S/C17H19FN2O2/c1-21-17-8-13(18)4-5-16(17)12-7-15(10-19-9-12)20-14-3-2-6-22-11-14/h4-5,7-10,14,20H,2-3,6,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344268
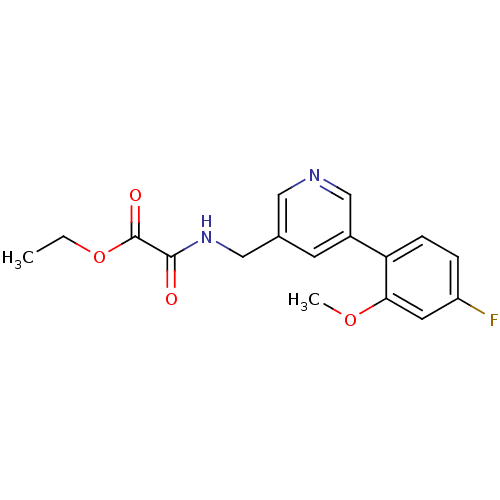
(CHEMBL1779020 | ethyl 2-((5-(4-fluoro-2-methoxyphe...)Show InChI InChI=1S/C17H17FN2O4/c1-3-24-17(22)16(21)20-9-11-6-12(10-19-8-11)14-5-4-13(18)7-15(14)23-2/h4-8,10H,3,9H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344246

(3-(6-((cyclopentylamino)methyl)pyrazin-2-yl)benzon...)Show InChI InChI=1S/C17H18N4/c18-9-13-4-3-5-14(8-13)17-12-19-10-16(21-17)11-20-15-6-1-2-7-15/h3-5,8,10,12,15,20H,1-2,6-7,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344247

(4-(6-((cyclopentylamino)methyl)pyrazin-2-yl)benzon...)Show InChI InChI=1S/C17H18N4/c18-9-13-5-7-14(8-6-13)17-12-19-10-16(21-17)11-20-15-3-1-2-4-15/h5-8,10,12,15,20H,1-4,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344248
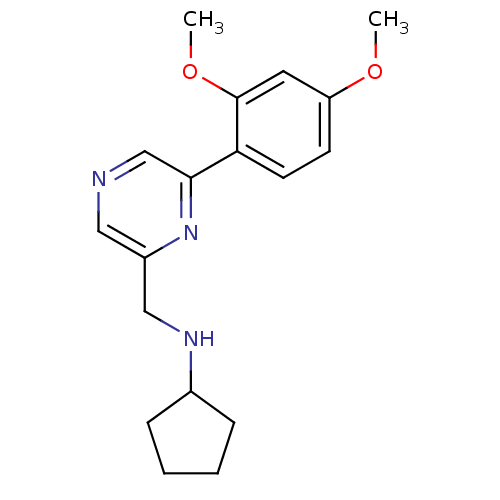
(CHEMBL1779001 | N-((6-(2,4-dimethoxyphenyl)pyrazin...)Show InChI InChI=1S/C18H23N3O2/c1-22-15-7-8-16(18(9-15)23-2)17-12-19-10-14(21-17)11-20-13-5-3-4-6-13/h7-10,12-13,20H,3-6,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344243
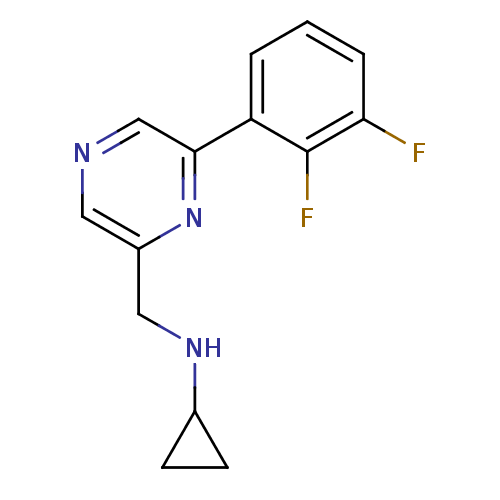
(CHEMBL1778996 | N-((6-(2,3-difluorophenyl)pyrazin-...)Show InChI InChI=1S/C14H13F2N3/c15-12-3-1-2-11(14(12)16)13-8-17-6-10(19-13)7-18-9-4-5-9/h1-3,6,8-9,18H,4-5,7H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344240

(1-(6-(4-fluoro-2-methoxyphenyl)pyrazin-2-yl)-N-(py...)Show InChI InChI=1S/C18H17FN4O/c1-24-18-8-13(19)5-6-16(18)17-12-21-11-15(23-17)10-20-9-14-4-2-3-7-22-14/h2-8,11-12,20H,9-10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344245
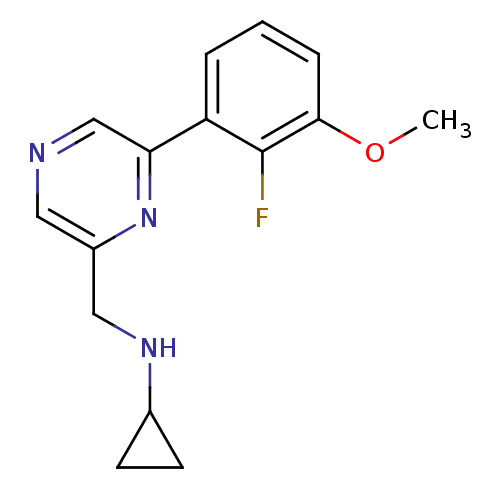
(CHEMBL1778998 | N-((6-(2-fluoro-3-methoxyphenyl)py...)Show InChI InChI=1S/C15H16FN3O/c1-20-14-4-2-3-12(15(14)16)13-9-17-7-11(19-13)8-18-10-5-6-10/h2-4,7,9-10,18H,5-6,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344253
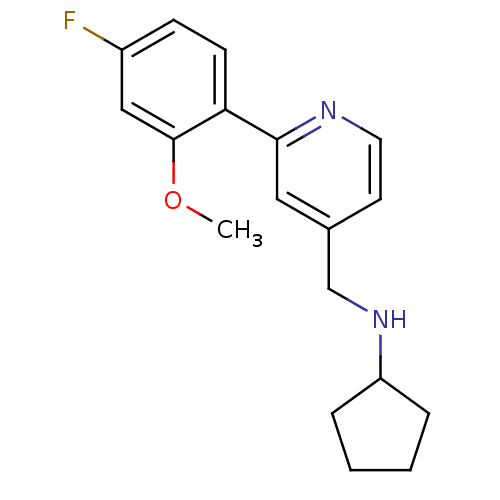
(CHEMBL1779006 | N-((2-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-11-14(19)6-7-16(18)17-10-13(8-9-20-17)12-21-15-4-2-3-5-15/h6-11,15,21H,2-5,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50344249

(CHEMBL1779002 | N-(3-(6-((cyclopentylamino)methyl)...)Show InChI InChI=1S/C18H22N4O/c1-13(23)21-16-8-4-5-14(9-16)18-12-19-10-17(22-18)11-20-15-6-2-3-7-15/h4-5,8-10,12,15,20H,2-3,6-7,11H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2A
(Homo sapiens (Human)) | BDBM50344263
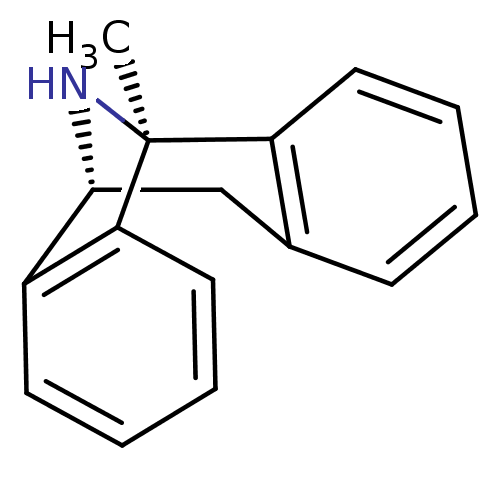
((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...)Show InChI InChI=1S/C16H15N/c1-16-13-8-4-2-6-11(13)10-15(17-16)12-7-3-5-9-14(12)16/h2-9,15,17H,10H2,1H3/t15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at NR2A transfected in oocytes |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM50032651

(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human NR2B expressed in HEK293 cells assessed as glutamate-induced changes in intracellular calcium concentration |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50344250

(CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-9-15(19)6-7-17(18)14-8-13(10-20-12-14)11-21-16-4-2-3-5-16/h6-10,12,16,21H,2-5,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM50344259

(CHEMBL1779012 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H15FN4O2/c1-25-17-7-14(19)2-3-15(17)13-6-12(8-21-10-13)9-23-18(24)16-11-20-4-5-22-16/h2-8,10-11H,9H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human NR2B expressed in HEK293 cells assessed as glutamate-induced changes in intracellular calcium concentration |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50344232

(1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...)Show InChI InChI=1S/C16H18FN3O/c1-21-16-6-12(17)4-5-14(16)15-10-19-9-13(20-15)8-18-7-11-2-3-11/h4-6,9-11,18H,2-3,7-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM50344232

(1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...)Show InChI InChI=1S/C16H18FN3O/c1-21-16-6-12(17)4-5-14(16)15-10-19-9-13(20-15)8-18-7-11-2-3-11/h4-6,9-11,18H,2-3,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 508 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human NR2B expressed in HEK293 cells assessed as glutamate-induced changes in intracellular calcium concentration |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM50344260

(CHEMBL1779013 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H16FN3O2/c1-25-18-9-15(20)5-6-16(18)14-8-13(10-21-12-14)11-23-19(24)17-4-2-3-7-22-17/h2-10,12H,11H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 673 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human NR2B expressed in HEK293 cells assessed as glutamate-induced changes in intracellular calcium concentration |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM50344250

(CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-9-15(19)6-7-17(18)14-8-13(10-20-12-14)11-21-16-4-2-3-5-16/h6-10,12,16,21H,2-5,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human NR2B expressed in HEK293 cells assessed as glutamate-induced changes in intracellular calcium concentration |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344232

(1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...)Show InChI InChI=1S/C16H18FN3O/c1-21-16-6-12(17)4-5-14(16)15-10-19-9-13(20-15)8-18-7-11-2-3-11/h4-6,9-11,18H,2-3,7-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344250

(CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-9-15(19)6-7-17(18)14-8-13(10-20-12-14)11-21-16-4-2-3-5-16/h6-10,12,16,21H,2-5,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344251

(CHEMBL1779004 | N-((4'-fluoro-2'-methoxybiphenyl-3...)Show InChI InChI=1S/C19H22FNO/c1-22-19-12-16(20)9-10-18(19)15-6-4-5-14(11-15)13-21-17-7-2-3-8-17/h4-6,9-12,17,21H,2-3,7-8,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344254

(CHEMBL1779007 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H23FN2O/c1-23-19-10-16(20)7-8-18(19)15-9-14(11-21-13-15)12-22-17-5-3-2-4-6-17/h7-11,13,17,22H,2-6,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344253

(CHEMBL1779006 | N-((2-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-11-14(19)6-7-16(18)17-10-13(8-9-20-17)12-21-15-4-2-3-5-15/h6-11,15,21H,2-5,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50032651

(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344257

(CHEMBL1779010 | N-benzyl-5-(4-fluoro-2-methoxyphen...)Show InChI InChI=1S/C19H17FN2O/c1-23-19-10-16(20)7-8-18(19)15-9-17(13-21-12-15)22-11-14-5-3-2-4-6-14/h2-10,12-13,22H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344252

(CHEMBL1779005 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-11-13(19)9-10-16(18)17-8-4-7-15(21-17)12-20-14-5-2-3-6-14/h4,7-11,14,20H,2-3,5-6,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344255

(CHEMBL1779008 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C16H19FN2O/c1-11(2)19-9-12-6-13(10-18-8-12)15-5-4-14(17)7-16(15)20-3/h4-8,10-11,19H,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344259

(CHEMBL1779012 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H15FN4O2/c1-25-17-7-14(19)2-3-15(17)13-6-12(8-21-10-13)9-23-18(24)16-11-20-4-5-22-16/h2-8,10-11H,9H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344256

(3-(4-fluoro-2-methoxyphenyl)-5-(pyrrolidin-2-yl)py...)Show InChI InChI=1S/C16H17FN2O/c1-20-16-8-13(17)4-5-14(16)11-7-12(10-18-9-11)15-3-2-6-19-15/h4-5,7-10,15,19H,2-3,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344258

(5-(4-fluoro-2-methoxyphenyl)-N-(tetrahydro-2H-pyra...)Show InChI InChI=1S/C17H19FN2O2/c1-21-17-8-13(18)4-5-16(17)12-7-15(10-19-9-12)20-14-3-2-6-22-11-14/h4-5,7-10,14,20H,2-3,6,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344234

(CHEMBL1778867 | N-((6-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C17H20FN3O/c1-22-17-8-12(18)6-7-15(17)16-11-19-9-14(21-16)10-20-13-4-2-3-5-13/h6-9,11,13,20H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344264

(CHEMBL1779016 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H19FN2O2/c1-23-17-8-15(19)5-6-16(17)14-7-12(9-20-11-14)10-21-18(22)13-3-2-4-13/h5-9,11,13H,2-4,10H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344261

(CHEMBL1779014 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C20H17FN2O2/c1-25-19-10-17(21)7-8-18(19)16-9-14(11-22-13-16)12-23-20(24)15-5-3-2-4-6-15/h2-11,13H,12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50344259

(CHEMBL1779012 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H15FN4O2/c1-25-17-7-14(19)2-3-15(17)13-6-12(8-21-10-13)9-23-18(24)16-11-20-4-5-22-16/h2-8,10-11H,9H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344232

(1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...)Show InChI InChI=1S/C16H18FN3O/c1-21-16-6-12(17)4-5-14(16)15-10-19-9-13(20-15)8-18-7-11-2-3-11/h4-6,9-11,18H,2-3,7-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344259

(CHEMBL1779012 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H15FN4O2/c1-25-17-7-14(19)2-3-15(17)13-6-12(8-21-10-13)9-23-18(24)16-11-20-4-5-22-16/h2-8,10-11H,9H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50032651

(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344260

(CHEMBL1779013 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H16FN3O2/c1-25-18-9-15(20)5-6-16(18)14-8-13(10-21-12-14)11-23-19(24)17-4-2-3-7-22-17/h2-10,12H,11H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50344260

(CHEMBL1779013 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H16FN3O2/c1-25-18-9-15(20)5-6-16(18)14-8-13(10-21-12-14)11-23-19(24)17-4-2-3-7-22-17/h2-10,12H,11H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344250

(CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C18H21FN2O/c1-22-18-9-15(19)6-7-17(18)14-8-13(10-20-12-14)11-21-16-4-2-3-5-16/h6-10,12,16,21H,2-5,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Iinhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344260

(CHEMBL1779013 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C19H16FN3O2/c1-25-18-9-15(20)5-6-16(18)14-8-13(10-21-12-14)11-23-19(24)17-4-2-3-7-22-17/h2-10,12H,11H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50344267

(CHEMBL1779019 | N-((5-(4-fluoro-2-methoxyphenyl)py...)Show InChI InChI=1S/C17H17FN2O2/c1-22-16-7-14(18)4-5-15(16)13-6-11(8-19-10-13)9-20-17(21)12-2-3-12/h4-8,10,12H,2-3,9H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells electrophysiology study |
Bioorg Med Chem Lett 21: 3399-403 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.117
BindingDB Entry DOI: 10.7270/Q29887BK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data