 Found 73 hits of Enzyme Inhibition Constant Data
Found 73 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344327
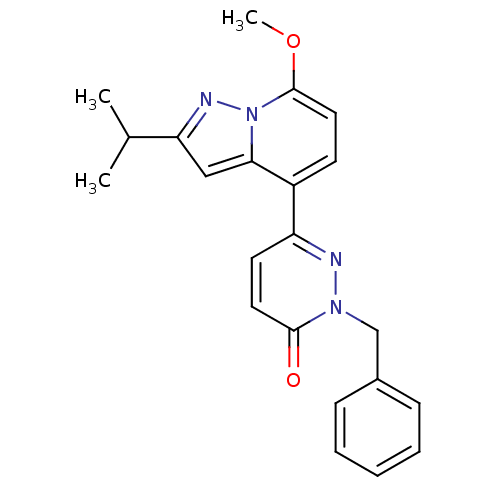
(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1ccc(-c2ccc(=O)n(Cc3ccccc3)n2)c2cc(nn12)C(C)C Show InChI InChI=1S/C22H22N4O2/c1-15(2)19-13-20-17(9-12-22(28-3)26(20)24-19)18-10-11-21(27)25(23-18)14-16-7-5-4-6-8-16/h4-13,15H,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344329

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1ccc(C2=NN(Cc3ccccc3)C(=O)CC2)c2cc(nn12)C(C)C |t:6| Show InChI InChI=1S/C22H24N4O2/c1-15(2)19-13-20-17(9-12-22(28-3)26(20)24-19)18-10-11-21(27)25(23-18)14-16-7-5-4-6-8-16/h4-9,12-13,15H,10-11,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344325
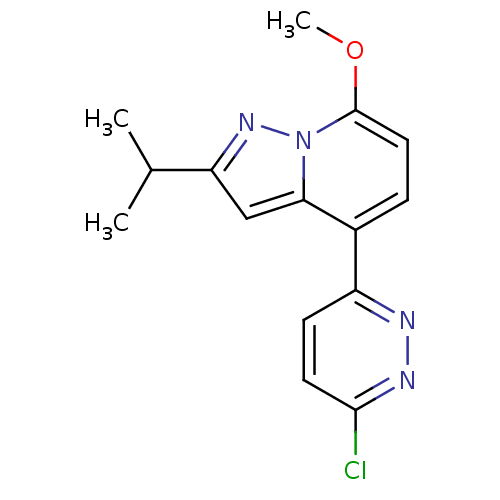
(4-(6-chloropyridazin-3-yl)-2-isopropyl-7-methoxypy...)Show InChI InChI=1S/C15H15ClN4O/c1-9(2)12-8-13-10(11-5-6-14(16)18-17-11)4-7-15(21-3)20(13)19-12/h4-9H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344321
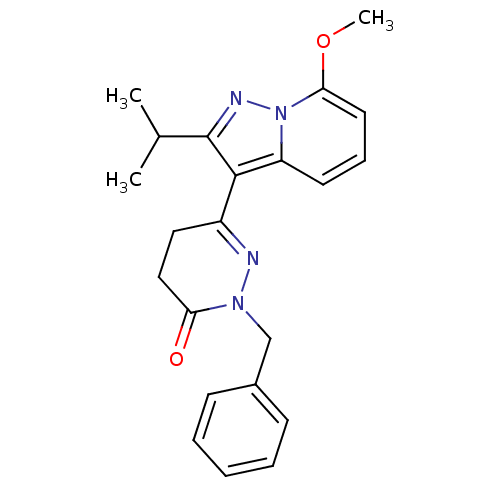
(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1cccc2c(c(nn12)C(C)C)C1=NN(Cc2ccccc2)C(=O)CC1 |t:16| Show InChI InChI=1S/C22H24N4O2/c1-15(2)22-21(18-10-7-11-20(28-3)26(18)24-22)17-12-13-19(27)25(23-17)14-16-8-5-4-6-9-16/h4-11,15H,12-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344314

(CHEMBL1779432 | rac-2-Benzyl-4a-methyl-4,4a,5,6-te...)Show SMILES CC12CCc3nn4ccccc4c3C1=NN(Cc1ccccc1)C(=O)C2 |c:16| Show InChI InChI=1S/C21H20N4O/c1-21-11-10-16-19(17-9-5-6-12-24(17)22-16)20(21)23-25(18(26)13-21)14-15-7-3-2-4-8-15/h2-9,12H,10-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344318
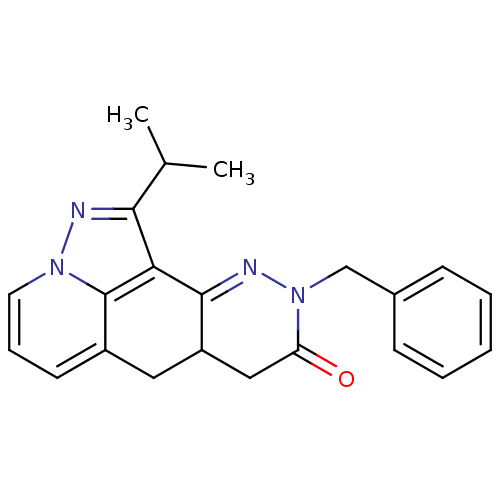
(CHEMBL1779436 | rac-9-Benzyl-1-isopropyl-6a,9,10b,...)Show SMILES CC(C)c1nn2cccc3CC4CC(=O)N(Cc5ccccc5)N=C4c1c23 |c:24| Show InChI InChI=1S/C22H22N4O/c1-14(2)20-19-21-17(11-16-9-6-10-25(23-20)22(16)19)12-18(27)26(24-21)13-15-7-4-3-5-8-15/h3-10,14,17H,11-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344326
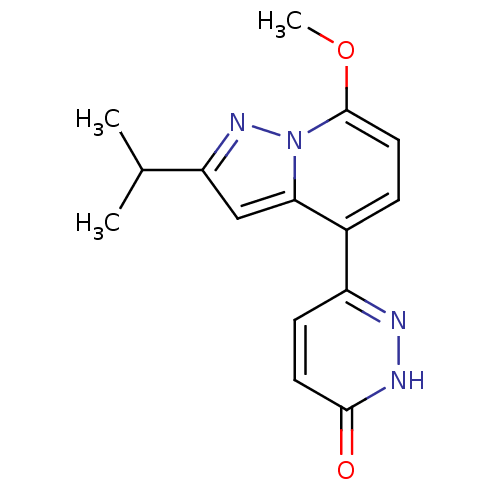
(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...)Show InChI InChI=1S/C15H16N4O2/c1-9(2)12-8-13-10(11-5-6-14(20)17-16-11)4-7-15(21-3)19(13)18-12/h4-9H,1-3H3,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344328

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...)Show SMILES COc1ccc(C2=NNC(=O)CC2)c2cc(nn12)C(C)C |t:6| Show InChI InChI=1S/C15H18N4O2/c1-9(2)12-8-13-10(11-5-6-14(20)17-16-11)4-7-15(21-3)19(13)18-12/h4,7-9H,5-6H2,1-3H3,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344326

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...)Show InChI InChI=1S/C15H16N4O2/c1-9(2)12-8-13-10(11-5-6-14(20)17-16-11)4-7-15(21-3)19(13)18-12/h4-9H,1-3H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344315
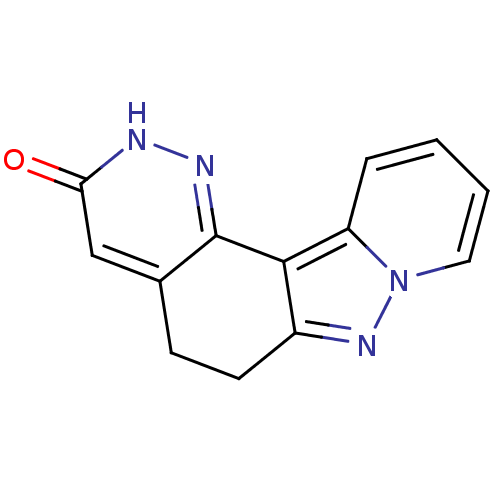
(5,6-Dihydro-2H-1,2,7,7a-tetraaza-benzo[c]fluoren-3...)Show InChI InChI=1S/C13H10N4O/c18-11-7-8-4-5-9-12(13(8)15-14-11)10-3-1-2-6-17(10)16-9/h1-3,6-7H,4-5H2,(H,14,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344306

((R)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...)Show SMILES CC(C)c1nn2ccccc2c1C1=NNC(=O)C[C@H]1C |r,t:14| Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50240404
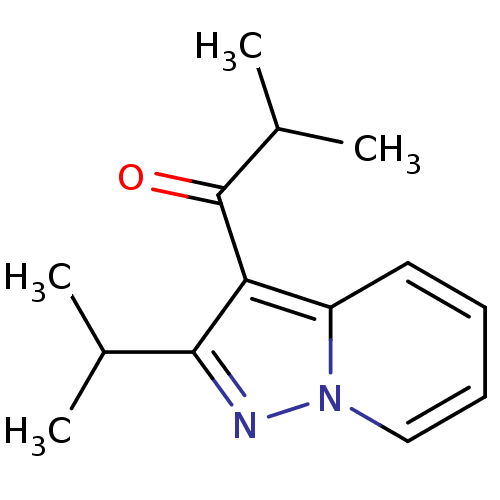
((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...)Show InChI InChI=1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344327

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1ccc(-c2ccc(=O)n(Cc3ccccc3)n2)c2cc(nn12)C(C)C Show InChI InChI=1S/C22H22N4O2/c1-15(2)19-13-20-17(9-12-22(28-3)26(20)24-19)18-10-11-21(27)25(23-18)14-16-7-5-4-6-8-16/h4-13,15H,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344311

(CHEMBL1779429 | rac-4,4a,5,6-Tetrahydro-2H-1,2,7,7...)Show InChI InChI=1S/C13H12N4O/c18-11-7-8-4-5-9-12(13(8)15-14-11)10-3-1-2-6-17(10)16-9/h1-3,6,8H,4-5,7H2,(H,14,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344314

(CHEMBL1779432 | rac-2-Benzyl-4a-methyl-4,4a,5,6-te...)Show SMILES CC12CCc3nn4ccccc4c3C1=NN(Cc1ccccc1)C(=O)C2 |c:16| Show InChI InChI=1S/C21H20N4O/c1-21-11-10-16-19(17-9-5-6-12-24(17)22-16)20(21)23-25(18(26)13-21)14-15-7-3-2-4-8-15/h2-9,12H,10-11,13-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344305

(CHEMBL1779335 | rac-6-(2-isopropylpyrazolo[1,5-a]p...)Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344312

(CHEMBL1779430 | rac-2-Benzyl-4,4a,5,6-tetrahydro-2...)Show SMILES O=C1CC2CCc3nn4ccccc4c3C2=NN1Cc1ccccc1 |c:18| Show InChI InChI=1S/C20H18N4O/c25-18-12-15-9-10-16-19(17-8-4-5-11-23(17)21-16)20(15)22-24(18)13-14-6-2-1-3-7-14/h1-8,11,15H,9-10,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344306

((R)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...)Show SMILES CC(C)c1nn2ccccc2c1C1=NNC(=O)C[C@H]1C |r,t:14| Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344313
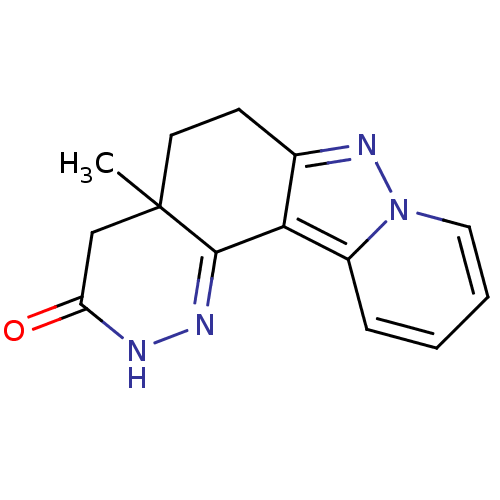
(CHEMBL1779431 | rac-4a-Methyl-4,4a,5,6-tetrahydro-...)Show InChI InChI=1S/C14H14N4O/c1-14-6-5-9-12(13(14)16-15-11(19)8-14)10-4-2-3-7-18(10)17-9/h2-4,7H,5-6,8H2,1H3,(H,15,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344316

(2-Benzyl-5,6-dihydro-2H-1,2,7,7a-tetraaza-benzo[c]...)Show InChI InChI=1S/C20H16N4O/c25-18-12-15-9-10-16-19(17-8-4-5-11-23(17)21-16)20(15)22-24(18)13-14-6-2-1-3-7-14/h1-8,11-12H,9-10,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344307

((S)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...)Show SMILES CC(C)c1nn2ccccc2c1C1=NNC(=O)C[C@@H]1C |r,t:14| Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344319

(2-benzyl-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl...)Show SMILES CC(C)c1nn2ccccc2c1C1=NN(Cc2ccccc2)C(=O)CC1 |t:14| Show InChI InChI=1S/C21H22N4O/c1-15(2)21-20(18-10-6-7-13-24(18)23-21)17-11-12-19(26)25(22-17)14-16-8-4-3-5-9-16/h3-10,13,15H,11-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344320

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-3-y...)Show SMILES COc1cccc2c(c(nn12)C(C)C)C1=NNC(=O)CC1 |t:16| Show InChI InChI=1S/C15H18N4O2/c1-9(2)15-14(10-7-8-12(20)17-16-10)11-5-4-6-13(21-3)19(11)18-15/h4-6,9H,7-8H2,1-3H3,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344306

((R)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...)Show SMILES CC(C)c1nn2ccccc2c1C1=NNC(=O)C[C@H]1C |r,t:14| Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344312

(CHEMBL1779430 | rac-2-Benzyl-4,4a,5,6-tetrahydro-2...)Show SMILES O=C1CC2CCc3nn4ccccc4c3C2=NN1Cc1ccccc1 |c:18| Show InChI InChI=1S/C20H18N4O/c25-18-12-15-9-10-16-19(17-8-4-5-11-23(17)21-16)20(15)22-24(18)13-14-6-2-1-3-7-14/h1-8,11,15H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344329

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1ccc(C2=NN(Cc3ccccc3)C(=O)CC2)c2cc(nn12)C(C)C |t:6| Show InChI InChI=1S/C22H24N4O2/c1-15(2)19-13-20-17(9-12-22(28-3)26(20)24-19)18-10-11-21(27)25(23-18)14-16-7-5-4-6-8-16/h4-9,12-13,15H,10-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344322
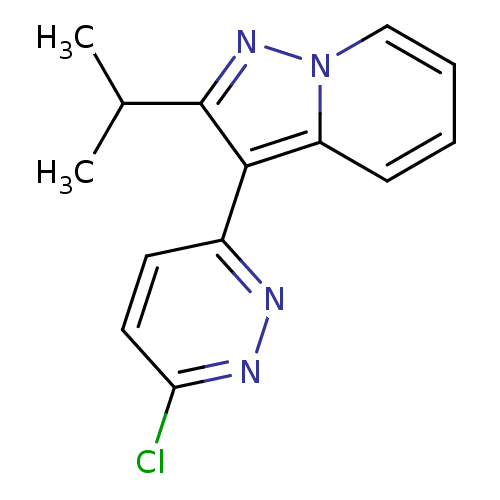
(3-(6-chloropyridazin-3-yl)-2-isopropylpyrazolo[1,5...)Show InChI InChI=1S/C14H13ClN4/c1-9(2)14-13(10-6-7-12(15)17-16-10)11-5-3-4-8-19(11)18-14/h3-9H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344313

(CHEMBL1779431 | rac-4a-Methyl-4,4a,5,6-tetrahydro-...)Show InChI InChI=1S/C14H14N4O/c1-14-6-5-9-12(13(14)16-15-11(19)8-14)10-4-2-3-7-18(10)17-9/h2-4,7H,5-6,8H2,1H3,(H,15,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344329

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1ccc(C2=NN(Cc3ccccc3)C(=O)CC2)c2cc(nn12)C(C)C |t:6| Show InChI InChI=1S/C22H24N4O2/c1-15(2)19-13-20-17(9-12-22(28-3)26(20)24-19)18-10-11-21(27)25(23-18)14-16-7-5-4-6-8-16/h4-9,12-13,15H,10-11,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344317

(CHEMBL1779435 | rac-1-Isopropyl-6a,9-dihydro-6H,7H...)Show InChI InChI=1S/C15H16N4O/c1-8(2)13-12-14-10(7-11(20)16-17-14)6-9-4-3-5-19(18-13)15(9)12/h3-5,8,10H,6-7H2,1-2H3,(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344328

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...)Show SMILES COc1ccc(C2=NNC(=O)CC2)c2cc(nn12)C(C)C |t:6| Show InChI InChI=1S/C15H18N4O2/c1-9(2)12-8-13-10(11-5-6-14(20)17-16-11)4-7-15(21-3)19(13)18-12/h4,7-9H,5-6H2,1-3H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344308

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-4,5-dih...)Show InChI InChI=1S/C14H16N4O/c1-9(2)14-13(10-6-7-12(19)16-15-10)11-5-3-4-8-18(11)17-14/h3-5,8-9H,6-7H2,1-2H3,(H,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344327

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1ccc(-c2ccc(=O)n(Cc3ccccc3)n2)c2cc(nn12)C(C)C Show InChI InChI=1S/C22H22N4O2/c1-15(2)19-13-20-17(9-12-22(28-3)26(20)24-19)18-10-11-21(27)25(23-18)14-16-7-5-4-6-8-16/h4-13,15H,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344305

(CHEMBL1779335 | rac-6-(2-isopropylpyrazolo[1,5-a]p...)Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344322

(3-(6-chloropyridazin-3-yl)-2-isopropylpyrazolo[1,5...)Show InChI InChI=1S/C14H13ClN4/c1-9(2)14-13(10-6-7-12(15)17-16-10)11-5-3-4-8-19(11)18-14/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344316

(2-Benzyl-5,6-dihydro-2H-1,2,7,7a-tetraaza-benzo[c]...)Show InChI InChI=1S/C20H16N4O/c25-18-12-15-9-10-16-19(17-8-4-5-11-23(17)21-16)20(15)22-24(18)13-14-6-2-1-3-7-14/h1-8,11-12H,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344309
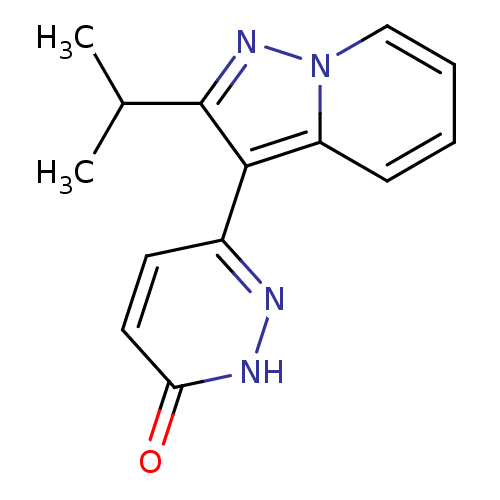
(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)pyridazi...)Show InChI InChI=1S/C14H14N4O/c1-9(2)14-13(10-6-7-12(19)16-15-10)11-5-3-4-8-18(11)17-14/h3-9H,1-2H3,(H,16,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344315

(5,6-Dihydro-2H-1,2,7,7a-tetraaza-benzo[c]fluoren-3...)Show InChI InChI=1S/C13H10N4O/c18-11-7-8-4-5-9-12(13(8)15-14-11)10-3-1-2-6-17(10)16-9/h1-3,6-7H,4-5H2,(H,14,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344308

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-4,5-dih...)Show InChI InChI=1S/C14H16N4O/c1-9(2)14-13(10-6-7-12(19)16-15-10)11-5-3-4-8-18(11)17-14/h3-5,8-9H,6-7H2,1-2H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344309

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)pyridazi...)Show InChI InChI=1S/C14H14N4O/c1-9(2)14-13(10-6-7-12(19)16-15-10)11-5-3-4-8-18(11)17-14/h3-9H,1-2H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344305

(CHEMBL1779335 | rac-6-(2-isopropylpyrazolo[1,5-a]p...)Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344326

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...)Show InChI InChI=1S/C15H16N4O2/c1-9(2)12-8-13-10(11-5-6-14(20)17-16-11)4-7-15(21-3)19(13)18-12/h4-9H,1-3H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50240404

((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...)Show InChI InChI=1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344308

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-4,5-dih...)Show InChI InChI=1S/C14H16N4O/c1-9(2)14-13(10-6-7-12(19)16-15-10)11-5-3-4-8-18(11)17-14/h3-5,8-9H,6-7H2,1-2H3,(H,16,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344309

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)pyridazi...)Show InChI InChI=1S/C14H14N4O/c1-9(2)14-13(10-6-7-12(19)16-15-10)11-5-3-4-8-18(11)17-14/h3-9H,1-2H3,(H,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344324

(2-isopropyl-3-(6-methoxypyridazin-3-yl)pyrazolo[1,...)Show InChI InChI=1S/C15H16N4O/c1-10(2)15-14(11-7-8-13(20-3)17-16-11)12-6-4-5-9-19(12)18-15/h4-10H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344311

(CHEMBL1779429 | rac-4,4a,5,6-Tetrahydro-2H-1,2,7,7...)Show InChI InChI=1S/C13H12N4O/c18-11-7-8-4-5-9-12(13(8)15-14-11)10-3-1-2-6-17(10)16-9/h1-3,6,8H,4-5,7H2,(H,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344315

(5,6-Dihydro-2H-1,2,7,7a-tetraaza-benzo[c]fluoren-3...)Show InChI InChI=1S/C13H10N4O/c18-11-7-8-4-5-9-12(13(8)15-14-11)10-3-1-2-6-17(10)16-9/h1-3,6-7H,4-5H2,(H,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344311

(CHEMBL1779429 | rac-4,4a,5,6-Tetrahydro-2H-1,2,7,7...)Show InChI InChI=1S/C13H12N4O/c18-11-7-8-4-5-9-12(13(8)15-14-11)10-3-1-2-6-17(10)16-9/h1-3,6,8H,4-5,7H2,(H,14,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344317

(CHEMBL1779435 | rac-1-Isopropyl-6a,9-dihydro-6H,7H...)Show InChI InChI=1S/C15H16N4O/c1-8(2)13-12-14-10(7-11(20)16-17-14)6-9-4-3-5-19(18-13)15(9)12/h3-5,8,10H,6-7H2,1-2H3,(H,16,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344320

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-3-y...)Show SMILES COc1cccc2c(c(nn12)C(C)C)C1=NNC(=O)CC1 |t:16| Show InChI InChI=1S/C15H18N4O2/c1-9(2)15-14(10-7-8-12(20)17-16-10)11-5-4-6-13(21-3)19(11)18-15/h4-6,9H,7-8H2,1-3H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344320

(6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-3-y...)Show SMILES COc1cccc2c(c(nn12)C(C)C)C1=NNC(=O)CC1 |t:16| Show InChI InChI=1S/C15H18N4O2/c1-9(2)15-14(10-7-8-12(20)17-16-10)11-5-4-6-13(21-3)19(11)18-15/h4-6,9H,7-8H2,1-3H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50240404

((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...)Show InChI InChI=1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 2.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344317

(CHEMBL1779435 | rac-1-Isopropyl-6a,9-dihydro-6H,7H...)Show InChI InChI=1S/C15H16N4O/c1-8(2)13-12-14-10(7-11(20)16-17-14)6-9-4-3-5-19(18-13)15(9)12/h3-5,8,10H,6-7H2,1-2H3,(H,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344323

(3-(6-chloro-4-methylpyridazin-3-yl)-2-isopropylpyr...)Show SMILES CC(C)c1nn2ccccc2c1-c1nnc(Cl)cc1C |(.96,-30.94,;.19,-32.27,;.95,-33.6,;-1.35,-32.26,;-2.26,-33.49,;-3.72,-33.01,;-5.05,-33.79,;-6.37,-33.01,;-6.37,-31.47,;-5.05,-30.71,;-3.71,-31.47,;-2.25,-31.01,;-1.75,-29.55,;-.24,-29.24,;.24,-27.79,;-.77,-26.63,;-.28,-25.17,;-2.28,-26.94,;-2.78,-28.4,;-4.29,-28.71,)| Show InChI InChI=1S/C15H15ClN4/c1-9(2)14-13(11-6-4-5-7-20(11)19-14)15-10(3)8-12(16)17-18-15/h4-9H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344310

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-methy...)Show SMILES CC(C)c1nn2ccccc2c1-c1n[nH]c(=O)cc1C |(24.14,-12.96,;23.37,-14.29,;24.13,-15.63,;21.83,-14.28,;20.92,-15.51,;19.46,-15.03,;18.13,-15.81,;16.81,-15.03,;16.81,-13.49,;18.13,-12.73,;19.47,-13.5,;20.93,-13.03,;21.43,-11.57,;22.94,-11.26,;23.42,-9.81,;22.41,-8.65,;22.9,-7.19,;20.9,-8.96,;20.4,-10.42,;18.89,-10.73,)| Show InChI InChI=1S/C15H16N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-9H,1-3H3,(H,16,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344323

(3-(6-chloro-4-methylpyridazin-3-yl)-2-isopropylpyr...)Show SMILES CC(C)c1nn2ccccc2c1-c1nnc(Cl)cc1C |(.96,-30.94,;.19,-32.27,;.95,-33.6,;-1.35,-32.26,;-2.26,-33.49,;-3.72,-33.01,;-5.05,-33.79,;-6.37,-33.01,;-6.37,-31.47,;-5.05,-30.71,;-3.71,-31.47,;-2.25,-31.01,;-1.75,-29.55,;-.24,-29.24,;.24,-27.79,;-.77,-26.63,;-.28,-25.17,;-2.28,-26.94,;-2.78,-28.4,;-4.29,-28.71,)| Show InChI InChI=1S/C15H15ClN4/c1-9(2)14-13(11-6-4-5-7-20(11)19-14)15-10(3)8-12(16)17-18-15/h4-9H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344307

((S)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...)Show SMILES CC(C)c1nn2ccccc2c1C1=NNC(=O)C[C@@H]1C |r,t:14| Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344324

(2-isopropyl-3-(6-methoxypyridazin-3-yl)pyrazolo[1,...)Show InChI InChI=1S/C15H16N4O/c1-10(2)15-14(11-7-8-13(20-3)17-16-11)12-6-4-5-9-19(12)18-15/h4-10H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344321

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1cccc2c(c(nn12)C(C)C)C1=NN(Cc2ccccc2)C(=O)CC1 |t:16| Show InChI InChI=1S/C22H24N4O2/c1-15(2)22-21(18-10-7-11-20(28-3)26(18)24-22)17-12-13-19(27)25(23-17)14-16-8-5-4-6-9-16/h4-11,15H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344318

(CHEMBL1779436 | rac-9-Benzyl-1-isopropyl-6a,9,10b,...)Show SMILES CC(C)c1nn2cccc3CC4CC(=O)N(Cc5ccccc5)N=C4c1c23 |c:24| Show InChI InChI=1S/C22H22N4O/c1-14(2)20-19-21-17(11-16-9-6-10-25(23-20)22(16)19)12-18(27)26(24-21)13-15-7-4-3-5-8-15/h3-10,14,17H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344319

(2-benzyl-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl...)Show SMILES CC(C)c1nn2ccccc2c1C1=NN(Cc2ccccc2)C(=O)CC1 |t:14| Show InChI InChI=1S/C21H22N4O/c1-15(2)21-20(18-10-6-7-13-24(18)23-21)17-11-12-19(26)25(22-17)14-16-8-4-3-5-9-16/h3-10,13,15H,11-12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344313

(CHEMBL1779431 | rac-4a-Methyl-4,4a,5,6-tetrahydro-...)Show InChI InChI=1S/C14H14N4O/c1-14-6-5-9-12(13(14)16-15-11(19)8-14)10-4-2-3-7-18(10)17-9/h2-4,7H,5-6,8H2,1H3,(H,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344310

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-methy...)Show SMILES CC(C)c1nn2ccccc2c1-c1n[nH]c(=O)cc1C |(24.14,-12.96,;23.37,-14.29,;24.13,-15.63,;21.83,-14.28,;20.92,-15.51,;19.46,-15.03,;18.13,-15.81,;16.81,-15.03,;16.81,-13.49,;18.13,-12.73,;19.47,-13.5,;20.93,-13.03,;21.43,-11.57,;22.94,-11.26,;23.42,-9.81,;22.41,-8.65,;22.9,-7.19,;20.9,-8.96,;20.4,-10.42,;18.89,-10.73,)| Show InChI InChI=1S/C15H16N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-9H,1-3H3,(H,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344321

(2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...)Show SMILES COc1cccc2c(c(nn12)C(C)C)C1=NN(Cc2ccccc2)C(=O)CC1 |t:16| Show InChI InChI=1S/C22H24N4O2/c1-15(2)22-21(18-10-7-11-20(28-3)26(18)24-22)17-12-13-19(27)25(23-17)14-16-8-5-4-6-9-16/h4-11,15H,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344316

(2-Benzyl-5,6-dihydro-2H-1,2,7,7a-tetraaza-benzo[c]...)Show InChI InChI=1S/C20H16N4O/c25-18-12-15-9-10-16-19(17-8-4-5-11-23(17)21-16)20(15)22-24(18)13-14-6-2-1-3-7-14/h1-8,11-12H,9-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344322

(3-(6-chloropyridazin-3-yl)-2-isopropylpyrazolo[1,5...)Show InChI InChI=1S/C14H13ClN4/c1-9(2)14-13(10-6-7-12(15)17-16-10)11-5-3-4-8-19(11)18-14/h3-9H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344307

((S)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...)Show SMILES CC(C)c1nn2ccccc2c1C1=NNC(=O)C[C@@H]1C |r,t:14| Show InChI InChI=1S/C15H18N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-7,9-10H,8H2,1-3H3,(H,16,20)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344312

(CHEMBL1779430 | rac-2-Benzyl-4,4a,5,6-tetrahydro-2...)Show SMILES O=C1CC2CCc3nn4ccccc4c3C2=NN1Cc1ccccc1 |c:18| Show InChI InChI=1S/C20H18N4O/c25-18-12-15-9-10-16-19(17-8-4-5-11-23(17)21-16)20(15)22-24(18)13-14-6-2-1-3-7-14/h1-8,11,15H,9-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344314

(CHEMBL1779432 | rac-2-Benzyl-4a-methyl-4,4a,5,6-te...)Show SMILES CC12CCc3nn4ccccc4c3C1=NN(Cc1ccccc1)C(=O)C2 |c:16| Show InChI InChI=1S/C21H20N4O/c1-21-11-10-16-19(17-9-5-6-12-24(17)22-16)20(21)23-25(18(26)13-21)14-15-7-3-2-4-8-15/h2-9,12H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50344323

(3-(6-chloro-4-methylpyridazin-3-yl)-2-isopropylpyr...)Show SMILES CC(C)c1nn2ccccc2c1-c1nnc(Cl)cc1C |(.96,-30.94,;.19,-32.27,;.95,-33.6,;-1.35,-32.26,;-2.26,-33.49,;-3.72,-33.01,;-5.05,-33.79,;-6.37,-33.01,;-6.37,-31.47,;-5.05,-30.71,;-3.71,-31.47,;-2.25,-31.01,;-1.75,-29.55,;-.24,-29.24,;.24,-27.79,;-.77,-26.63,;-.28,-25.17,;-2.28,-26.94,;-2.78,-28.4,;-4.29,-28.71,)| Show InChI InChI=1S/C15H15ClN4/c1-9(2)14-13(11-6-4-5-7-20(11)19-14)15-10(3)8-12(16)17-18-15/h4-9H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50344310

(6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-methy...)Show SMILES CC(C)c1nn2ccccc2c1-c1n[nH]c(=O)cc1C |(24.14,-12.96,;23.37,-14.29,;24.13,-15.63,;21.83,-14.28,;20.92,-15.51,;19.46,-15.03,;18.13,-15.81,;16.81,-15.03,;16.81,-13.49,;18.13,-12.73,;19.47,-13.5,;20.93,-13.03,;21.43,-11.57,;22.94,-11.26,;23.42,-9.81,;22.41,-8.65,;22.9,-7.19,;20.9,-8.96,;20.4,-10.42,;18.89,-10.73,)| Show InChI InChI=1S/C15H16N4O/c1-9(2)14-13(11-6-4-5-7-19(11)18-14)15-10(3)8-12(20)16-17-15/h4-9H,1-3H3,(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50344324

(2-isopropyl-3-(6-methoxypyridazin-3-yl)pyrazolo[1,...)Show InChI InChI=1S/C15H16N4O/c1-10(2)15-14(11-7-8-13(20-3)17-16-11)12-6-4-5-9-19(12)18-15/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate |
Bioorg Med Chem Lett 21: 3307-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.021
BindingDB Entry DOI: 10.7270/Q2X067C3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data