 Found 27 hits of Enzyme Inhibition Constant Data
Found 27 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347092
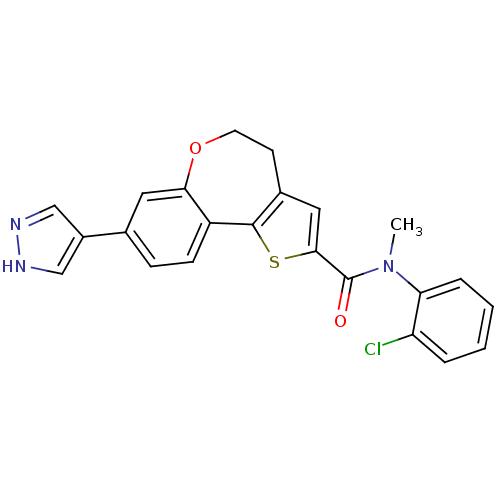
(CHEMBL1796757)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)-c1cn[nH]c1)c1ccccc1Cl Show InChI InChI=1S/C23H18ClN3O2S/c1-27(19-5-3-2-4-18(19)24)23(28)21-11-15-8-9-29-20-10-14(16-12-25-26-13-16)6-7-17(20)22(15)30-21/h2-7,10-13H,8-9H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347089
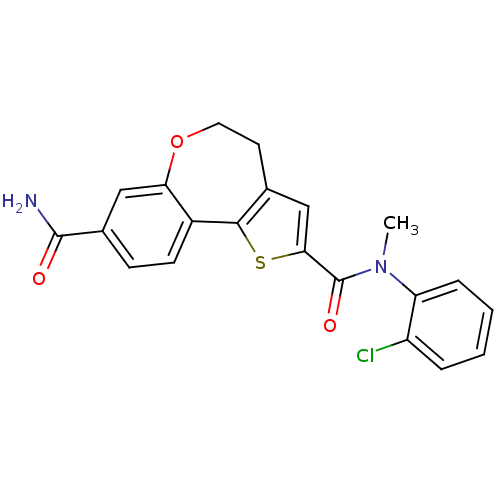
(CHEMBL1796275)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-10-13(20(23)25)6-7-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347088
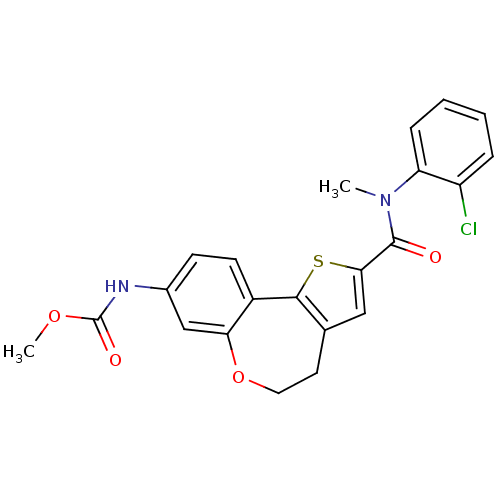
(CHEMBL1796274)Show SMILES COC(=O)Nc1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O4S/c1-25(17-6-4-3-5-16(17)23)21(26)19-11-13-9-10-29-18-12-14(24-22(27)28-2)7-8-15(18)20(13)30-19/h3-8,11-12H,9-10H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087
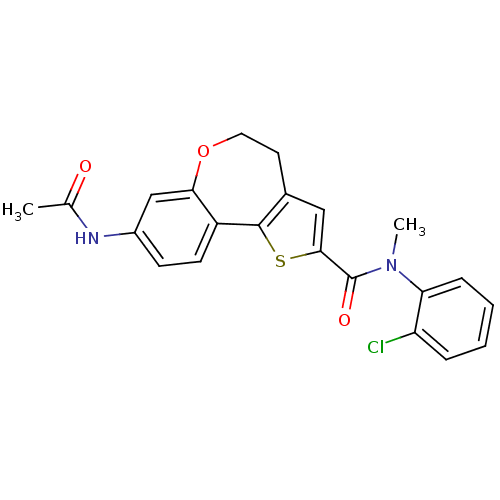
(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347095

(CHEMBL1796760)Show SMILES CNC(=O)c1ccc(N(C)C(=O)c2cc3CCOc4cc(ccc4-c3s2)C(=O)NC)c(Cl)c1 Show InChI InChI=1S/C24H22ClN3O4S/c1-26-22(29)14-5-7-18(17(25)10-14)28(3)24(31)20-12-13-8-9-32-19-11-15(23(30)27-2)4-6-16(19)21(13)33-20/h4-7,10-12H,8-9H2,1-3H3,(H,26,29)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347090
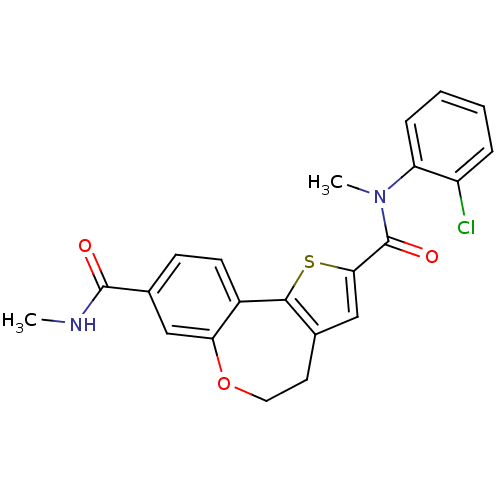
(CHEMBL1796276)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-24-21(26)14-7-8-15-18(11-14)28-10-9-13-12-19(29-20(13)15)22(27)25(2)17-6-4-3-5-16(17)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347085

(CHEMBL1796271)Show InChI InChI=1S/C20H16ClNO2S/c1-22(16-8-4-3-7-15(16)21)20(23)18-12-13-10-11-24-17-9-5-2-6-14(17)19(13)25-18/h2-9,12H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347091

(CHEMBL1796756)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C(=O)N1CCOCC1)c1ccccc1Cl Show InChI InChI=1S/C25H23ClN2O4S/c1-27(20-5-3-2-4-19(20)26)25(30)22-15-16-8-11-32-21-14-17(6-7-18(21)23(16)33-22)24(29)28-9-12-31-13-10-28/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347094

(CHEMBL1796759)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)-c1cn[nH]c1)c1ccncc1Cl Show InChI InChI=1S/C22H17ClN4O2S/c1-27(18-4-6-24-12-17(18)23)22(28)20-9-14-5-7-29-19-8-13(15-10-25-26-11-15)2-3-16(19)21(14)30-20/h2-4,6,8-12H,5,7H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347093
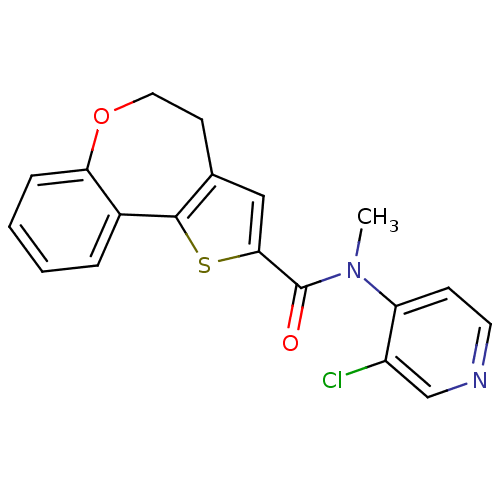
(CHEMBL1796758)Show InChI InChI=1S/C19H15ClN2O2S/c1-22(15-6-8-21-11-14(15)20)19(23)17-10-12-7-9-24-16-5-3-2-4-13(16)18(12)25-17/h2-6,8,10-11H,7,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 473 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347085

(CHEMBL1796271)Show InChI InChI=1S/C20H16ClNO2S/c1-22(16-8-4-3-7-15(16)21)20(23)18-12-13-10-11-24-17-9-5-2-6-14(17)19(13)25-18/h2-9,12H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347081
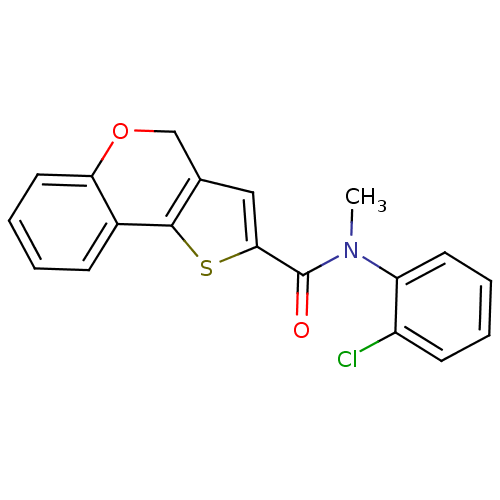
(CHEMBL1796267)Show InChI InChI=1S/C19H14ClNO2S/c1-21(15-8-4-3-7-14(15)20)19(22)17-10-12-11-23-16-9-5-2-6-13(16)18(12)24-17/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347080

(CHEMBL1796266)Show InChI InChI=1S/C20H17NO3S/c1-21(15-8-4-6-10-17(15)23-2)20(22)18-11-13-12-24-16-9-5-3-7-14(16)19(13)25-18/h3-11H,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 673 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347079

(CHEMBL1796265)Show InChI InChI=1S/C20H17NO2S/c1-13-7-3-5-9-16(13)21(2)20(22)18-11-14-12-23-17-10-6-4-8-15(17)19(14)24-18/h3-11H,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347077
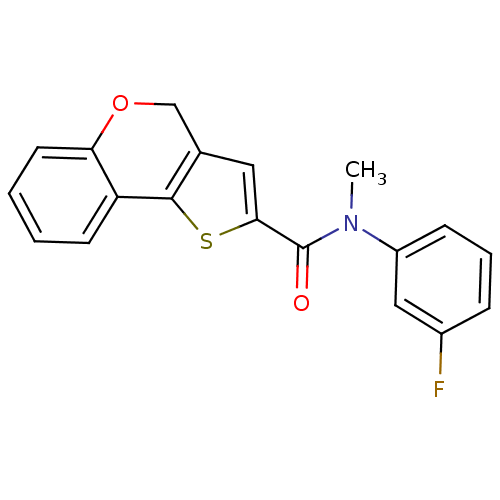
(CHEMBL1796263)Show InChI InChI=1S/C19H14FNO2S/c1-21(14-6-4-5-13(20)10-14)19(22)17-9-12-11-23-16-8-3-2-7-15(16)18(12)24-17/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347078
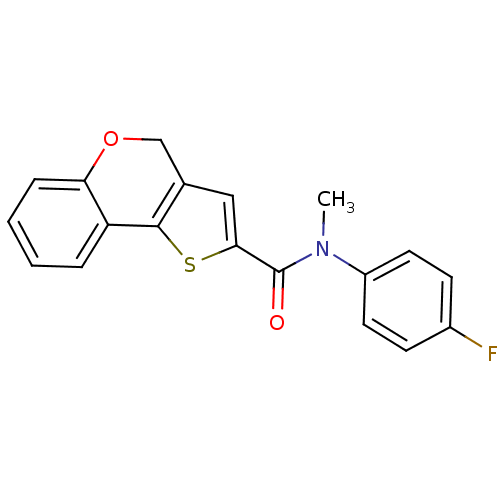
(CHEMBL1796264)Show InChI InChI=1S/C19H14FNO2S/c1-21(14-8-6-13(20)7-9-14)19(22)17-10-12-11-23-16-5-3-2-4-15(16)18(12)24-17/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347083
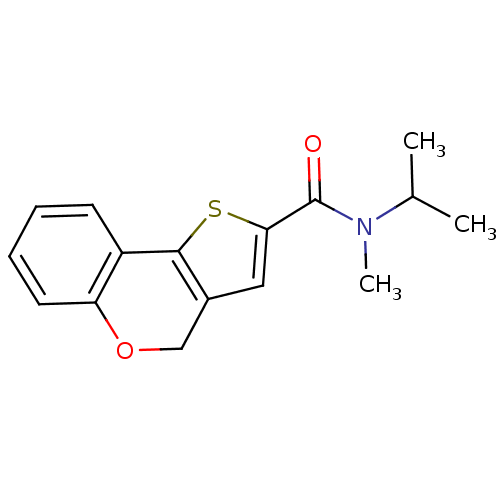
(CHEMBL1796269)Show InChI InChI=1S/C16H17NO2S/c1-10(2)17(3)16(18)14-8-11-9-19-13-7-5-4-6-12(13)15(11)20-14/h4-8,10H,9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347086
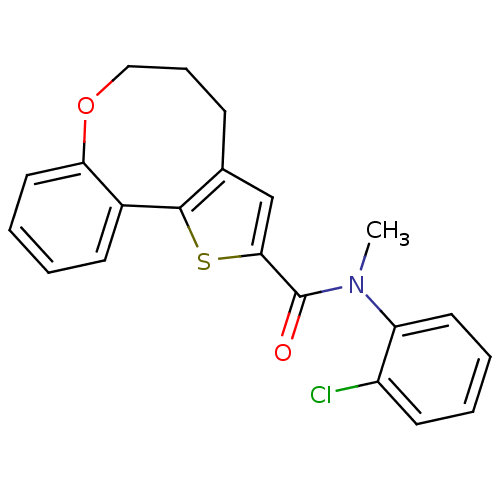
(CHEMBL1796272)Show InChI InChI=1S/C21H18ClNO2S/c1-23(17-10-4-3-9-16(17)22)21(24)19-13-14-7-6-12-25-18-11-5-2-8-15(18)20(14)26-19/h2-5,8-11,13H,6-7,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347082
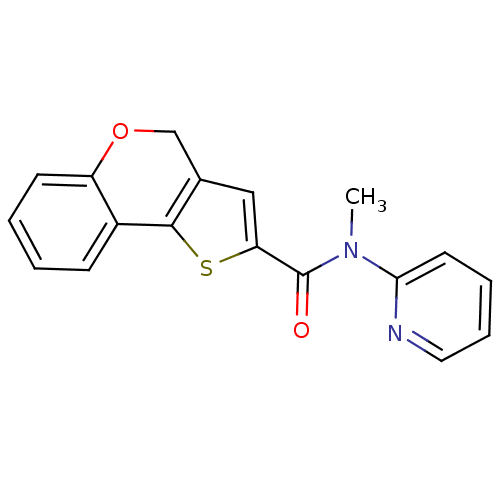
(CHEMBL1796268)Show InChI InChI=1S/C18H14N2O2S/c1-20(16-8-4-5-9-19-16)18(21)15-10-12-11-22-14-7-3-2-6-13(14)17(12)23-15/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347084

(CHEMBL1796270)Show InChI InChI=1S/C17H19NO2S/c1-11(2)9-18(3)17(19)15-8-12-10-20-14-7-5-4-6-13(14)16(12)21-15/h4-8,11H,9-10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data