 Found 51 hits of Enzyme Inhibition Constant Data
Found 51 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ATP-citrate synthase
(Rattus norvegicus) | BDBM50036210

((2S,3S)-3-Carboxy-2,3-dihydroxy-pentanedioic acid ...)Show InChI InChI=1S/C6H8O8/c7-2(8)1-6(14,5(12)13)3(9)4(10)11/h3,9,14H,1H2,(H,7,8)(H,10,11)(H,12,13)/t3-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394668
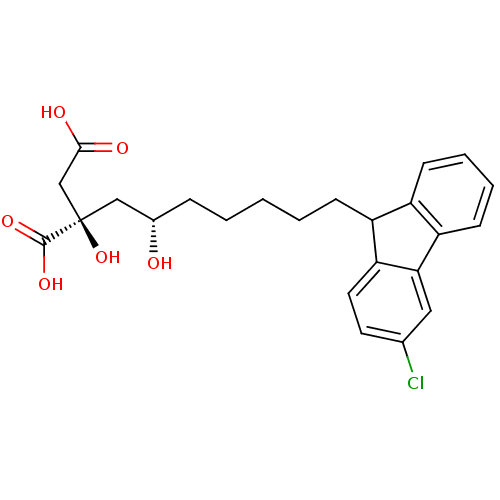
(CHEMBL2163366)Show SMILES O[C@@H](CCCCCC1c2ccccc2-c2cc(Cl)ccc12)C[C@@](O)(CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H27ClO6/c25-15-10-11-20-18(17-8-4-5-9-19(17)21(20)12-15)7-3-1-2-6-16(26)13-24(31,23(29)30)14-22(27)28/h4-5,8-12,16,18,26,31H,1-3,6-7,13-14H2,(H,27,28)(H,29,30)/t16-,18?,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM24567

((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...)Show SMILES CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@H](CC(C)C)NC=O |r| Show InChI InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant FASN thioesterase domain by fluorigenic assay |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394671
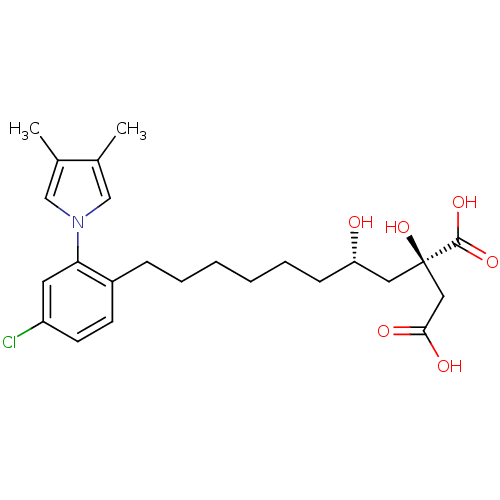
(CHEMBL2165260)Show SMILES Cc1cn(cc1C)-c1cc(Cl)ccc1CCCCCC[C@H](O)C[C@@](O)(CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H32ClNO6/c1-16-14-26(15-17(16)2)21-11-19(25)10-9-18(21)7-5-3-4-6-8-20(27)12-24(32,23(30)31)13-22(28)29/h9-11,14-15,20,27,32H,3-8,12-13H2,1-2H3,(H,28,29)(H,30,31)/t20-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394671

(CHEMBL2165260)Show SMILES Cc1cn(cc1C)-c1cc(Cl)ccc1CCCCCC[C@H](O)C[C@@](O)(CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H32ClNO6/c1-16-14-26(15-17(16)2)21-11-19(25)10-9-18(21)7-5-3-4-6-8-20(27)12-24(32,23(30)31)13-22(28)29/h9-11,14-15,20,27,32H,3-8,12-13H2,1-2H3,(H,28,29)(H,30,31)/t20-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394670
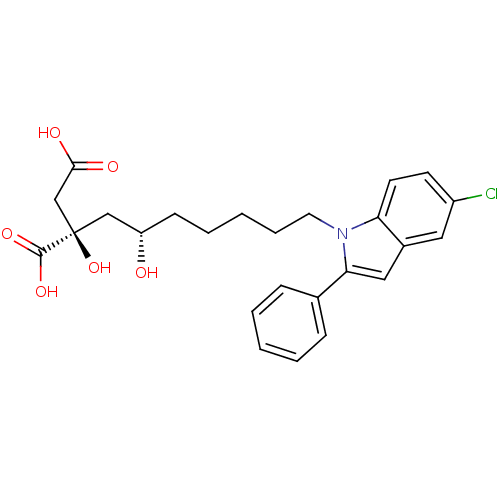
(CHEMBL2165263)Show SMILES O[C@@H](CCCCCn1c(cc2cc(Cl)ccc12)-c1ccccc1)C[C@@](O)(CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C25H28ClNO6/c26-19-10-11-21-18(13-19)14-22(17-7-3-1-4-8-17)27(21)12-6-2-5-9-20(28)15-25(33,24(31)32)16-23(29)30/h1,3-4,7-8,10-11,13-14,20,28,33H,2,5-6,9,12,15-16H2,(H,29,30)(H,31,32)/t20-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM24984
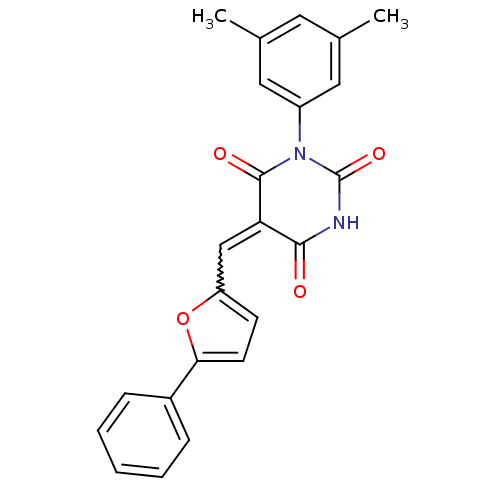
((5E)-1-(3,5-dimethylphenyl)-5-[(5-phenylfuran-2-yl...)Show SMILES Cc1cc(C)cc(c1)N1C(=O)NC(=O)C(=Cc2ccc(o2)-c2ccccc2)C1=O |w:15.16| Show InChI InChI=1S/C23H18N2O4/c1-14-10-15(2)12-17(11-14)25-22(27)19(21(26)24-23(25)28)13-18-8-9-20(29-18)16-6-4-3-5-7-16/h3-13H,1-2H3,(H,24,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant FASN thioesterase domain by fluorigenic assay |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50066676
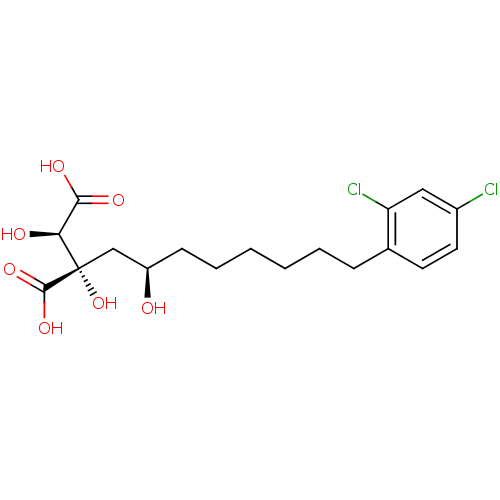
((2S,3R)-2-[(R)-8-(2,4-Dichloro-phenyl)-2-hydroxy-o...)Show SMILES O[C@H](CCCCCCc1ccc(Cl)cc1Cl)C[C@](O)([C@@H](O)C(O)=O)C(O)=O Show InChI InChI=1S/C18H24Cl2O7/c19-12-8-7-11(14(20)9-12)5-3-1-2-4-6-13(21)10-18(27,17(25)26)15(22)16(23)24/h7-9,13,15,21-22,27H,1-6,10H2,(H,23,24)(H,25,26)/t13-,15+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394671

(CHEMBL2165260)Show SMILES Cc1cn(cc1C)-c1cc(Cl)ccc1CCCCCC[C@H](O)C[C@@](O)(CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H32ClNO6/c1-16-14-26(15-17(16)2)21-11-19(25)10-9-18(21)7-5-3-4-6-8-20(27)12-24(32,23(30)31)13-22(28)29/h9-11,14-15,20,27,32H,3-8,12-13H2,1-2H3,(H,28,29)(H,30,31)/t20-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394672
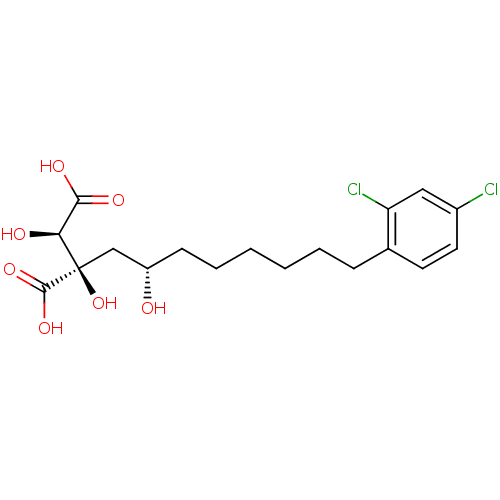
(CHEMBL2165259)Show SMILES O[C@@H](CCCCCCc1ccc(Cl)cc1Cl)C[C@@](O)([C@@H](O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H24Cl2O7/c19-12-8-7-11(14(20)9-12)5-3-1-2-4-6-13(21)10-18(27,17(25)26)15(22)16(23)24/h7-9,13,15,21-22,27H,1-6,10H2,(H,23,24)(H,25,26)/t13-,15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50394678
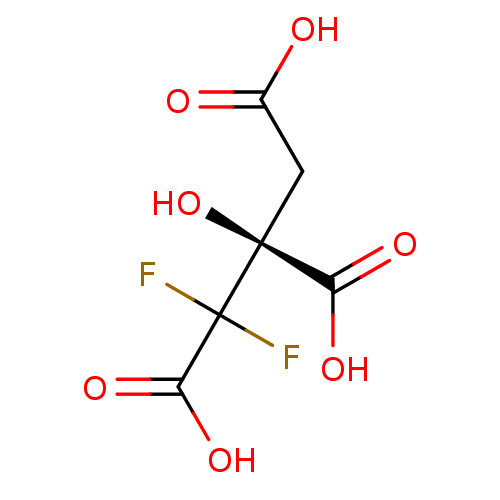
(CHEMBL2165261)Show InChI InChI=1S/C6H6F2O7/c7-6(8,4(13)14)5(15,3(11)12)1-2(9)10/h15H,1H2,(H,9,10)(H,11,12)(H,13,14)/t5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50394669
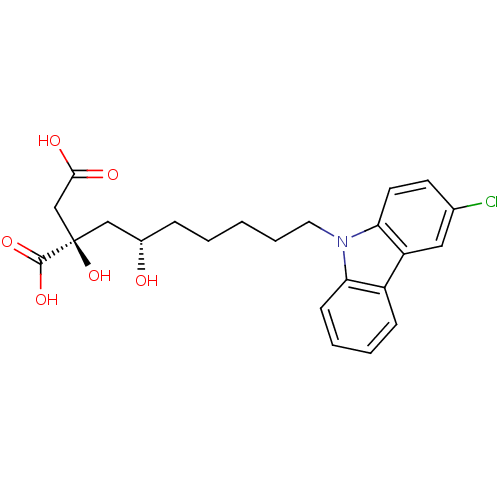
(CHEMBL2165264)Show SMILES O[C@@H](CCCCCn1c2ccccc2c2cc(Cl)ccc12)C[C@@](O)(CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H26ClNO6/c24-15-9-10-20-18(12-15)17-7-3-4-8-19(17)25(20)11-5-1-2-6-16(26)13-23(31,22(29)30)14-21(27)28/h3-4,7-10,12,16,26,31H,1-2,5-6,11,13-14H2,(H,27,28)(H,29,30)/t16-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50066683
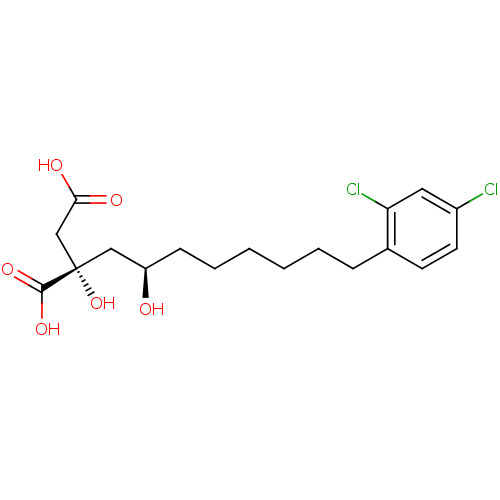
((S)-2-((R)-8-(2,4-dichlorophenyl)-2-hydroxyoctyl)-...)Show SMILES O[C@H](CCCCCCc1ccc(Cl)cc1Cl)C[C@](O)(CC(O)=O)C(O)=O Show InChI InChI=1S/C18H24Cl2O6/c19-13-8-7-12(15(20)9-13)5-3-1-2-4-6-14(21)10-18(26,17(24)25)11-16(22)23/h7-9,14,21,26H,1-6,10-11H2,(H,22,23)(H,24,25)/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50066683

((S)-2-((R)-8-(2,4-dichlorophenyl)-2-hydroxyoctyl)-...)Show SMILES O[C@H](CCCCCCc1ccc(Cl)cc1Cl)C[C@](O)(CC(O)=O)C(O)=O Show InChI InChI=1S/C18H24Cl2O6/c19-13-8-7-12(15(20)9-13)5-3-1-2-4-6-14(21)10-18(26,17(24)25)11-16(22)23/h7-9,14,21,26H,1-6,10-11H2,(H,22,23)(H,24,25)/t14-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50394664

(CHEMBL2165413)Show InChI InChI=1S/C18H18O2/c19-18-16(13-15-9-5-2-6-10-15)17(20-18)12-11-14-7-3-1-4-8-14/h1-10,16-17H,11-13H2/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant FASN thioesterase domain by fluorigenic assay |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50018569

(3,3,14,14-Tetramethyl-hexadecanedioic acid | CHEMB...)Show InChI InChI=1S/C20H38O4/c1-19(2,15-17(21)22)13-11-9-7-5-6-8-10-12-14-20(3,4)16-18(23)24/h5-16H2,1-4H3,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat liver ACLY using CoA as substrate by spectrophotometric analysis |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM15361
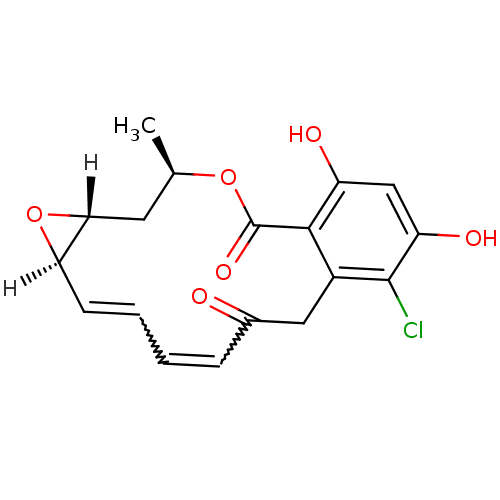
((1aR,2Z,4E,14R,15aR)-8-chloro-9,11-dihydroxy-14-me...)Show SMILES [H][C@@]12C[C@@H](C)OC(=O)c3c(O)cc(O)c(Cl)c3CC(=O)C=CC=C[C@@]1([H])O2 |r,w:22.22,20.20| Show InChI InChI=1S/C18H17ClO6/c1-9-6-15-14(25-15)5-3-2-4-10(20)7-11-16(18(23)24-9)12(21)8-13(22)17(11)19/h2-5,8-9,14-15,21-22H,6-7H2,1H3/t9-,14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat liver ACLY using ATP as substrate by spectrophotometric analysis |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50036210

((2S,3S)-3-Carboxy-2,3-dihydroxy-pentanedioic acid ...)Show InChI InChI=1S/C6H8O8/c7-2(8)1-6(14,5(12)13)3(9)4(10)11/h3,9,14H,1H2,(H,7,8)(H,10,11)(H,12,13)/t3-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY by fluorimetric analysis |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-citrate synthase
(Rattus norvegicus) | BDBM15361

((1aR,2Z,4E,14R,15aR)-8-chloro-9,11-dihydroxy-14-me...)Show SMILES [H][C@@]12C[C@@H](C)OC(=O)c3c(O)cc(O)c(Cl)c3CC(=O)C=CC=C[C@@]1([H])O2 |r,w:22.22,20.20| Show InChI InChI=1S/C18H17ClO6/c1-9-6-15-14(25-15)5-3-2-4-10(20)7-11-16(18(23)24-9)12(21)8-13(22)17(11)19/h2-5,8-9,14-15,21-22H,6-7H2,1H3/t9-,14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat liver ACLY using citrate as substrate by spectrophotometric analysis |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50394677
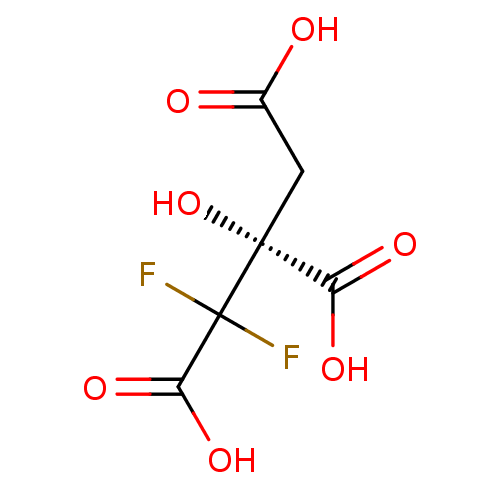
(CHEMBL2165262)Show InChI InChI=1S/C6H6F2O7/c7-6(8,4(13)14)5(15,3(11)12)1-2(9)10/h15H,1H2,(H,9,10)(H,11,12)(H,13,14)/t5-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50018569

(3,3,14,14-Tetramethyl-hexadecanedioic acid | CHEMB...)Show InChI InChI=1S/C20H38O4/c1-19(2,15-17(21)22)13-11-9-7-5-6-8-10-12-14-20(3,4)16-18(23)24/h5-16H2,1-4H3,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat liver ACLY using citrate as substrate by spectrophotometric analysis |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50394676

(CHEMBL2165252)Show InChI InChI=1S/C6H6O7/c7-2(8)1-6(5(11)12)3(13-6)4(9)10/h3H,1H2,(H,7,8)(H,9,10)(H,11,12)/t3-,6+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50394667
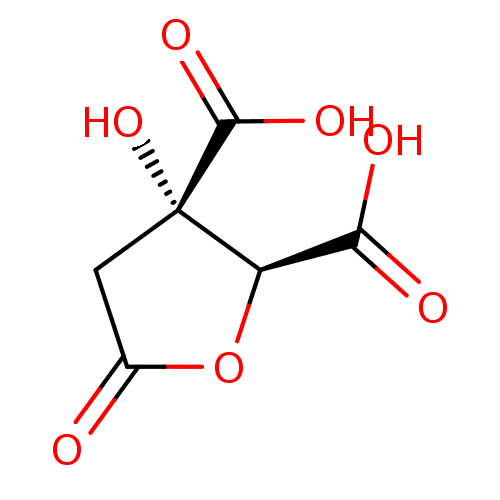
(CHEMBL2165253)Show InChI InChI=1S/C6H6O7/c7-2-1-6(12,5(10)11)3(13-2)4(8)9/h3,12H,1H2,(H,8,9)(H,10,11)/t3-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY by fluorimetric analysis |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50368516
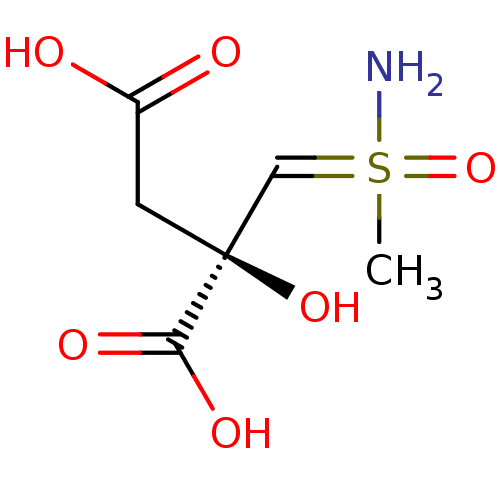
(CHEMBL1627232)Show InChI InChI=1S/C6H11NO6S/c1-14(7,13)3-6(12,5(10)11)2-4(8)9/h3,12H,2H2,1H3,(H2,7,13)(H,8,9)(H,10,11)/t6-,14?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50394661

(CHEMBL2165265)Show SMILES Cc1ccnc(NS(=O)(=O)c2ccc(NC(=O)Nc3ccc(cc3)S(=O)(=O)Nc3nccc(C)n3)cc2)n1 Show InChI InChI=1S/C23H22N8O5S2/c1-15-11-13-24-21(26-15)30-37(33,34)19-7-3-17(4-8-19)28-23(32)29-18-5-9-20(10-6-18)38(35,36)31-22-25-14-12-16(2)27-22/h3-14H,1-2H3,(H,24,26,30)(H,25,27,31)(H2,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant FASN ketoreductase site |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50394674
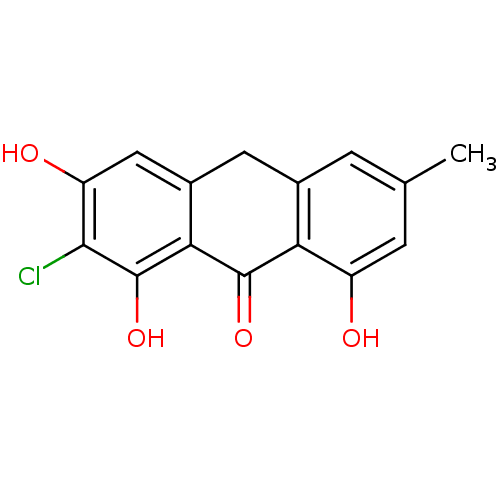
(CHEMBL2165254)Show InChI InChI=1S/C15H11ClO4/c1-6-2-7-4-8-5-10(18)13(16)15(20)12(8)14(19)11(7)9(17)3-6/h2-3,5,17-18,20H,4H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50394658

(CHEMBL2165411)Show SMILES CC(C)Oc1ccc(cc1)C1=NC(CSc2nc3ncccc3o2)CO1 |t:11| Show InChI InChI=1S/C19H19N3O3S/c1-12(2)24-15-7-5-13(6-8-15)18-21-14(10-23-18)11-26-19-22-17-16(25-19)4-3-9-20-17/h3-9,12,14H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl coA as substrate and [14C]NaHCO3 incubated for 5 mins prior to substrate addition by liquid scintillation count... |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50362887
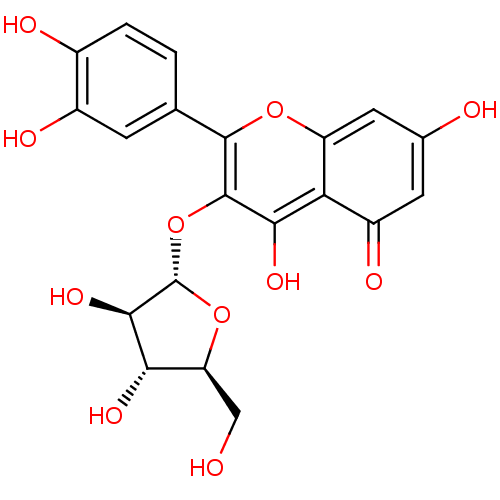
(AVICULARIN)Show SMILES OC[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H18O11/c21-6-13-15(26)17(28)20(30-13)31-19-16(27)14-11(25)4-8(22)5-12(14)29-18(19)7-1-2-9(23)10(24)3-7/h1-5,13,15,17,20-24,26-28H,6H2/t13-,15-,17+,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Rattus norvegicus) | BDBM50394675
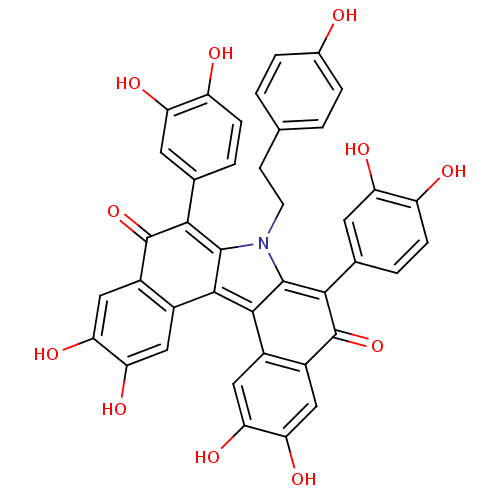
(PURPURONE)Show SMILES Oc1ccc(CCn2c3=C(C(=O)c4cc(O)c(O)cc4-c3c3-c4cc(O)c(O)cc4C(=O)C(c4ccc(O)c(O)c4)=c23)c2ccc(O)c(O)c2)cc1 |t:8,44| Show InChI InChI=1S/C40H27NO11/c42-20-5-1-17(2-6-20)9-10-41-37-33(18-3-7-25(43)27(45)11-18)39(51)23-15-31(49)29(47)13-21(23)35(37)36-22-14-30(48)32(50)16-24(22)40(52)34(38(36)41)19-4-8-26(44)28(46)12-19/h1-8,11-16,42-50H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver ACLY |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50318454

(CHEMBL123040 | Diallyl trisulfide | diallyltrisulf...)Show InChI InChI=1S/C6H10S3/c1-3-5-7-9-8-6-4-2/h3-4H,1-2,5-6H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50394660
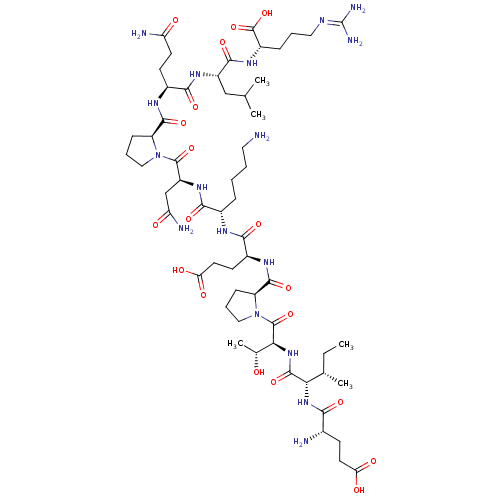
(CHEMBL2165409)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H97N17O19/c1-6-29(4)44(71-46(82)31(59)16-20-42(78)79)53(89)72-45(30(5)75)55(91)74-25-11-15-39(74)52(88)67-34(18-21-43(80)81)48(84)65-32(12-7-8-22-58)47(83)70-37(27-41(61)77)54(90)73-24-10-14-38(73)51(87)66-33(17-19-40(60)76)49(85)69-36(26-28(2)3)50(86)68-35(56(92)93)13-9-23-64-57(62)63/h28-39,44-45,75H,6-27,58-59H2,1-5H3,(H2,60,76)(H2,61,77)(H,65,84)(H,66,87)(H,67,88)(H,68,86)(H,69,85)(H,70,83)(H,71,82)(H,72,89)(H,78,79)(H,80,81)(H,92,93)(H4,62,63,64)/t29-,30+,31-,32-,33-,34-,35-,36-,37-,38-,39-,44-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FASN thioesterase domain |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
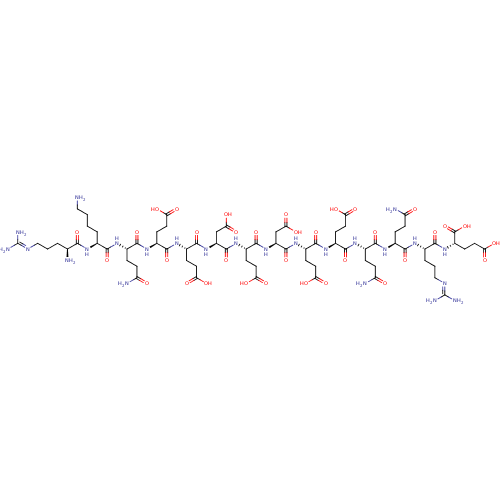
(Homo sapiens (Human)) | BDBM50394659
(CHEMBL2165410)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C71H114N24O34/c72-26-2-1-6-32(83-56(115)31(73)5-3-27-81-70(77)78)57(116)85-35(9-18-46(75)97)61(120)88-38(12-21-49(101)102)63(122)90-40(14-23-51(105)106)65(124)94-44(30-55(113)114)68(127)92-41(15-24-52(107)108)66(125)95-43(29-54(111)112)67(126)91-39(13-22-50(103)104)64(123)89-37(11-20-48(99)100)62(121)87-36(10-19-47(76)98)60(119)86-34(8-17-45(74)96)59(118)84-33(7-4-28-82-71(79)80)58(117)93-42(69(128)129)16-25-53(109)110/h31-44H,1-30,72-73H2,(H2,74,96)(H2,75,97)(H2,76,98)(H,83,115)(H,84,118)(H,85,116)(H,86,119)(H,87,121)(H,88,120)(H,89,123)(H,90,122)(H,91,126)(H,92,127)(H,93,117)(H,94,124)(H,95,125)(H,99,100)(H,101,102)(H,103,104)(H,105,106)(H,107,108)(H,109,110)(H,111,112)(H,113,114)(H,128,129)(H4,77,78,81)(H4,79,80,82)/t31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FASN thioesterase domain |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50394659

(CHEMBL2165410)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C71H114N24O34/c72-26-2-1-6-32(83-56(115)31(73)5-3-27-81-70(77)78)57(116)85-35(9-18-46(75)97)61(120)88-38(12-21-49(101)102)63(122)90-40(14-23-51(105)106)65(124)94-44(30-55(113)114)68(127)92-41(15-24-52(107)108)66(125)95-43(29-54(111)112)67(126)91-39(13-22-50(103)104)64(123)89-37(11-20-48(99)100)62(121)87-36(10-19-47(76)98)60(119)86-34(8-17-45(74)96)59(118)84-33(7-4-28-82-71(79)80)58(117)93-42(69(128)129)16-25-53(109)110/h31-44H,1-30,72-73H2,(H2,74,96)(H2,75,97)(H2,76,98)(H,83,115)(H,84,118)(H,85,116)(H,86,119)(H,87,121)(H,88,120)(H,89,123)(H,90,122)(H,91,126)(H,92,127)(H,93,117)(H,94,124)(H,95,125)(H,99,100)(H,101,102)(H,103,104)(H,105,106)(H,107,108)(H,109,110)(H,111,112)(H,113,114)(H,128,129)(H4,77,78,81)(H4,79,80,82)/t31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50009248
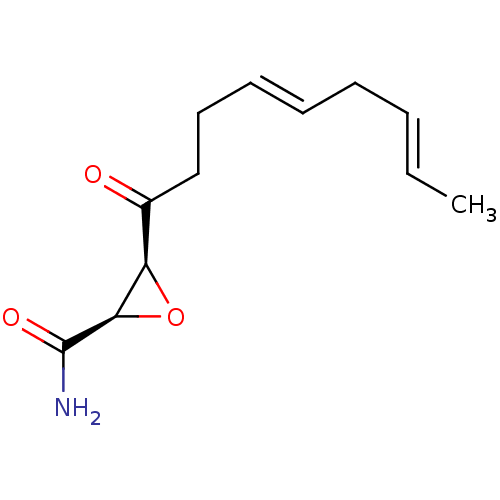
((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...)Show InChI InChI=1S/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human A375 cells |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50394660

(CHEMBL2165409)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H97N17O19/c1-6-29(4)44(71-46(82)31(59)16-20-42(78)79)53(89)72-45(30(5)75)55(91)74-25-11-15-39(74)52(88)67-34(18-21-43(80)81)48(84)65-32(12-7-8-22-58)47(83)70-37(27-41(61)77)54(90)73-24-10-14-38(73)51(87)66-33(17-19-40(60)76)49(85)69-36(26-28(2)3)50(86)68-35(56(92)93)13-9-23-64-57(62)63/h28-39,44-45,75H,6-27,58-59H2,1-5H3,(H2,60,76)(H2,61,77)(H,65,84)(H,66,87)(H,67,88)(H,68,86)(H,69,85)(H,70,83)(H,71,82)(H,72,89)(H,78,79)(H,80,81)(H,92,93)(H4,62,63,64)/t29-,30+,31-,32-,33-,34-,35-,36-,37-,38-,39-,44-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50256128

((2R,3S)-4-methylene-2-octyl-5-oxotetrahydrofuran-3...)Show InChI InChI=1S/C14H22O4/c1-3-4-5-6-7-8-9-11-12(13(15)16)10(2)14(17)18-11/h11-12H,2-9H2,1H3,(H,15,16)/t11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human A375 cells |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50153015

((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN after 3 hrs |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50256128

((2R,3S)-4-methylene-2-octyl-5-oxotetrahydrofuran-3...)Show InChI InChI=1S/C14H22O4/c1-3-4-5-6-7-8-9-11-12(13(15)16)10(2)14(17)18-11/h11-12H,2-9H2,1H3,(H,15,16)/t11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FASN thioesterase domain |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50153015

((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50394663
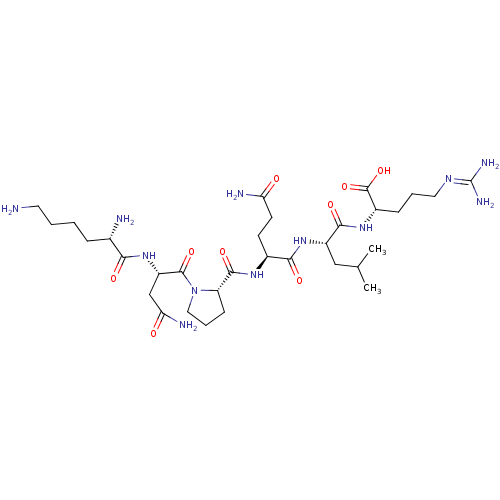
(CHEMBL2165267)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C32H58N12O9/c1-17(2)15-21(28(49)41-20(31(52)53)8-5-13-39-32(37)38)42-27(48)19(10-11-24(35)45)40-29(50)23-9-6-14-44(23)30(51)22(16-25(36)46)43-26(47)18(34)7-3-4-12-33/h17-23H,3-16,33-34H2,1-2H3,(H2,35,45)(H2,36,46)(H,40,50)(H,41,49)(H,42,48)(H,43,47)(H,52,53)(H4,37,38,39)/t18-,19-,20-,21-,22-,23-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50256128

((2R,3S)-4-methylene-2-octyl-5-oxotetrahydrofuran-3...)Show InChI InChI=1S/C14H22O4/c1-3-4-5-6-7-8-9-11-12(13(15)16)10(2)14(17)18-11/h11-12H,2-9H2,1H3,(H,15,16)/t11-,12+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50394665
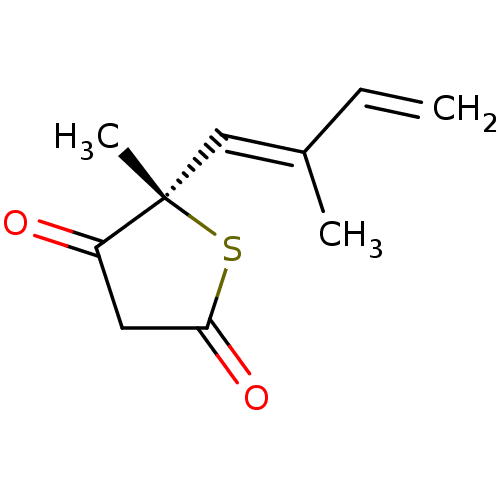
(CHEMBL251750)Show InChI InChI=1S/C10H12O2S/c1-4-7(2)6-10(3)8(11)5-9(12)13-10/h4,6H,1,5H2,2-3H3/b7-6+/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human HepG2 cells |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50394662
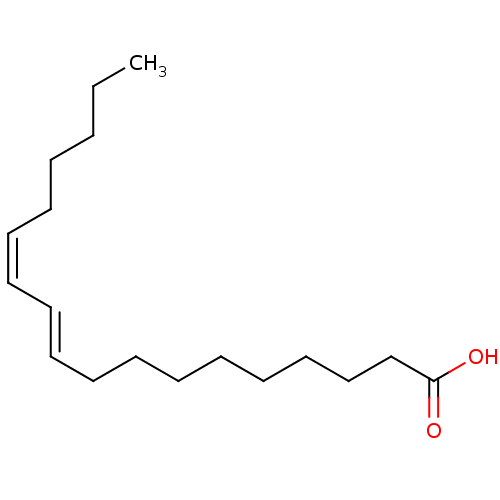
(CHEMBL1093743)Show InChI InChI=1S/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h6-9H,2-5,10-17H2,1H3,(H,19,20)/b7-6-,9-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human SKBR3 cells |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50394658

(CHEMBL2165411)Show SMILES CC(C)Oc1ccc(cc1)C1=NC(CSc2nc3ncccc3o2)CO1 |t:11| Show InChI InChI=1S/C19H19N3O3S/c1-12(2)24-15-7-5-13(6-8-15)18-21-14(10-23-18)11-26-19-22-17-16(25-19)4-3-9-20-17/h3-9,12,14H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl coA as substrate and [14C]NaHCO3 incubated for 5 mins prior to substrate addition by liquid scintillation count... |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Gallus gallus (Chicken)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of chicken liver FASN ketoreductase activity |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50394666
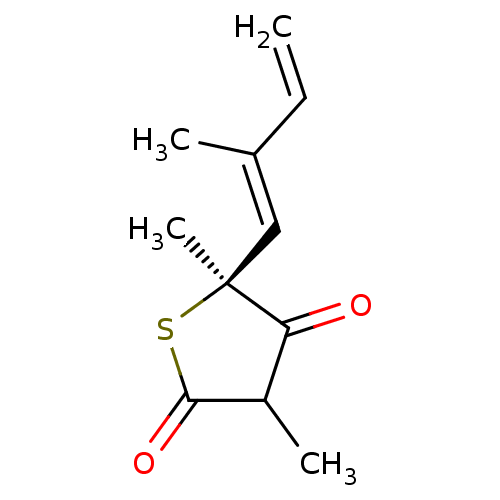
(CHEMBL248705)Show InChI InChI=1S/C11H14O2S/c1-5-7(2)6-11(4)9(12)8(3)10(13)14-11/h5-6,8H,1H2,2-4H3/b7-6+/t8?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human HepG2 cells |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50241313
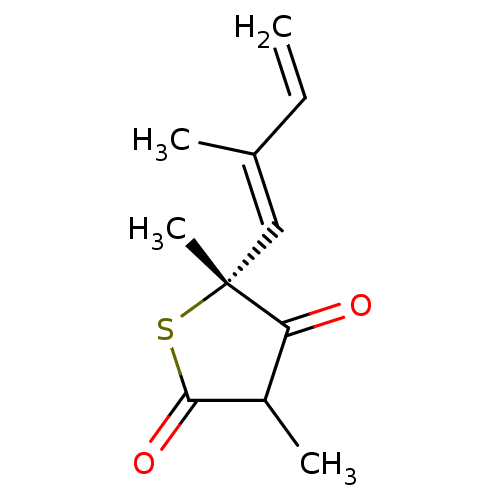
((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...)Show InChI InChI=1S/C11H14O2S/c1-5-7(2)6-11(4)9(12)8(3)10(13)14-11/h5-6,8H,1H2,2-4H3/b7-6+/t8?,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human HepG2 cells |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Rattus norvegicus) | BDBM50241313

((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...)Show InChI InChI=1S/C11H14O2S/c1-5-7(2)6-11(4)9(12)8(3)10(13)14-11/h5-6,8H,1H2,2-4H3/b7-6+/t8?,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Inhibition of FASN in rat liver |
J Med Chem 54: 5615-38 (2011)
Article DOI: 10.1021/jm2005805
BindingDB Entry DOI: 10.7270/Q21N8261 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data