 Found 85 hits of Enzyme Inhibition Constant Data
Found 85 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM25150
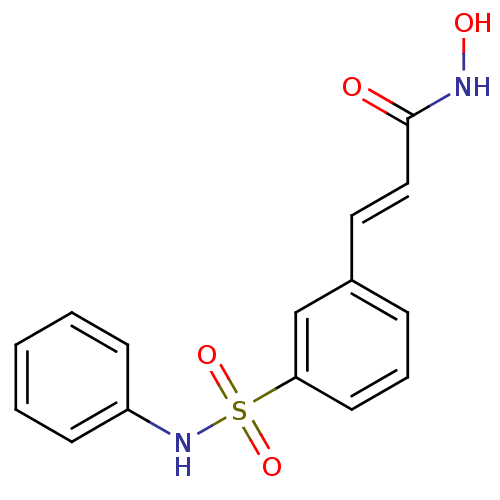
((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349504

(CHEMBL1808644)Show SMILES ONC(=O)\C=C\c1ccc2CN(CCc3c[nH]c4ccccc34)Cc2c1 Show InChI InChI=1S/C21H21N3O2/c25-21(23-26)8-6-15-5-7-17-13-24(14-18(17)11-15)10-9-16-12-22-20-4-2-1-3-19(16)20/h1-8,11-12,22,26H,9-10,13-14H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349485

(CHEMBL1808625)Show SMILES Cc1[nH]c2ccccc2c1CC(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H21N3O3/c1-14-19(18-4-2-3-5-20(18)23-14)11-22(27)25-12-16-8-6-15(10-17(16)13-25)7-9-21(26)24-28/h2-10,23,28H,11-13H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314631

(CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H23N3O2/c1-15-19(20-4-2-3-5-21(20)23-15)10-11-25-13-17-8-6-16(12-18(17)14-25)7-9-22(26)24-27/h2-9,12,23,27H,10-11,13-14H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349500

(CHEMBL1808640)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O5S/c1-25-16-5-7-17(8-6-16)26(23,24)20-11-14-4-2-13(10-15(14)12-20)3-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349495
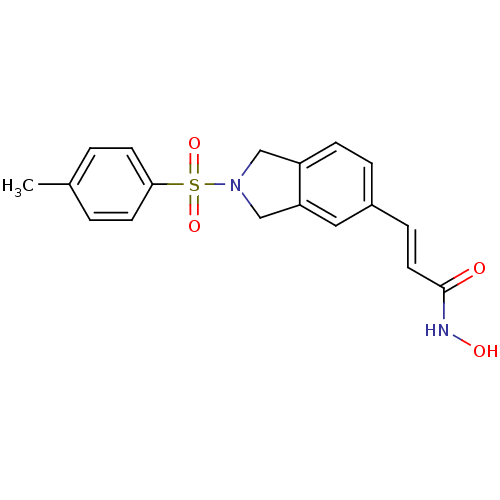
(CHEMBL1808635)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O4S/c1-13-2-7-17(8-3-13)25(23,24)20-11-15-6-4-14(10-16(15)12-20)5-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349496
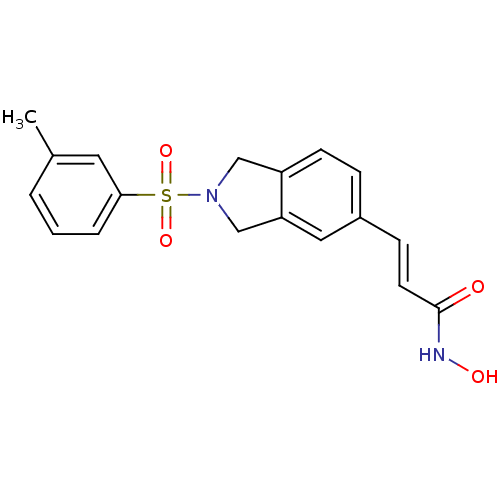
(CHEMBL1808636)Show SMILES Cc1cccc(c1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O4S/c1-13-3-2-4-17(9-13)25(23,24)20-11-15-7-5-14(10-16(15)12-20)6-8-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349505

(CHEMBL1808645)Show InChI InChI=1S/C20H20N4O2/c25-20(22-26)7-5-15-4-6-17-13-23(14-18(17)11-15)10-8-16-12-21-24-9-2-1-3-19(16)24/h1-7,9,11-12,26H,8,10,13-14H2,(H,22,25)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349491
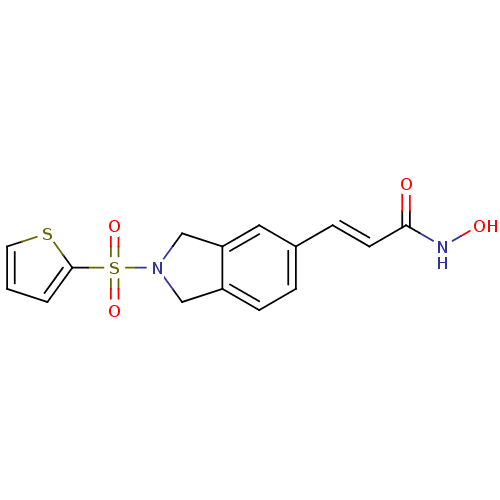
(CHEMBL1808631)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C15H14N2O4S2/c18-14(16-19)6-4-11-3-5-12-9-17(10-13(12)8-11)23(20,21)15-2-1-7-22-15/h1-8,19H,9-10H2,(H,16,18)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349501

(CHEMBL1808641)Show SMILES COc1cccc(c1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O5S/c1-25-16-3-2-4-17(10-16)26(23,24)20-11-14-7-5-13(9-15(14)12-20)6-8-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349503

(CHEMBL1808643)Show InChI InChI=1S/C19H26N2O2/c22-19(20-23)9-7-16-6-8-17-13-21(14-18(17)12-16)11-10-15-4-2-1-3-5-15/h6-9,12,15,23H,1-5,10-11,13-14H2,(H,20,22)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349489
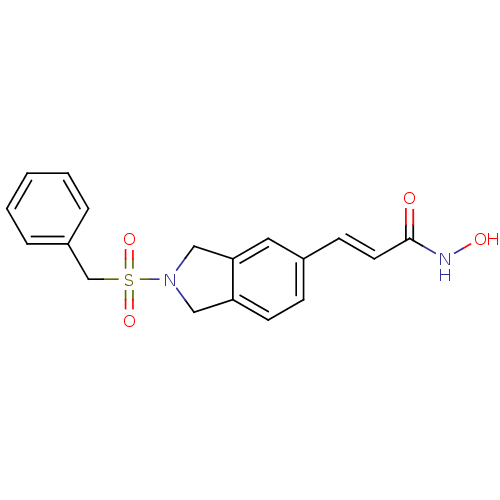
(CHEMBL1808629)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C18H18N2O4S/c21-18(19-22)9-7-14-6-8-16-11-20(12-17(16)10-14)25(23,24)13-15-4-2-1-3-5-15/h1-10,22H,11-13H2,(H,19,21)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349497
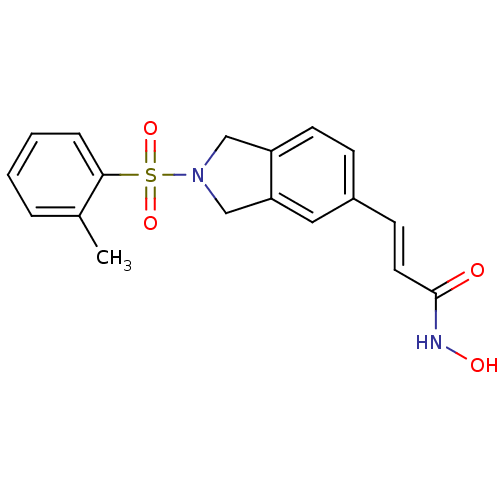
(CHEMBL1808637)Show SMILES Cc1ccccc1S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O4S/c1-13-4-2-3-5-17(13)25(23,24)20-11-15-8-6-14(10-16(15)12-20)7-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349484
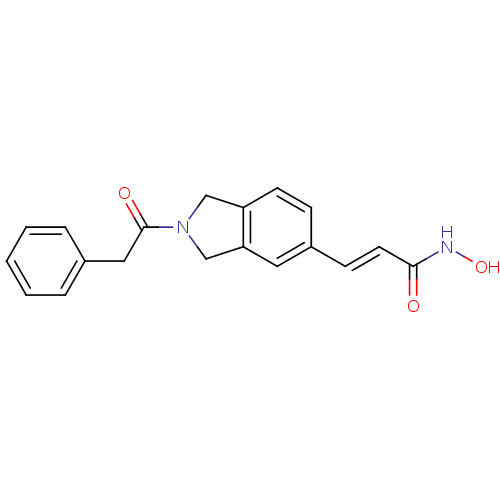
(CHEMBL1808624)Show InChI InChI=1S/C19H18N2O3/c22-18(20-24)9-7-15-6-8-16-12-21(13-17(16)10-15)19(23)11-14-4-2-1-3-5-14/h1-10,24H,11-13H2,(H,20,22)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349494

(CHEMBL1808634)Show SMILES Cn1cnc(c1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C15H16N4O4S/c1-18-9-15(16-10-18)24(22,23)19-7-12-4-2-11(6-13(12)8-19)3-5-14(20)17-21/h2-6,9-10,21H,7-8H2,1H3,(H,17,20)/b5-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349487
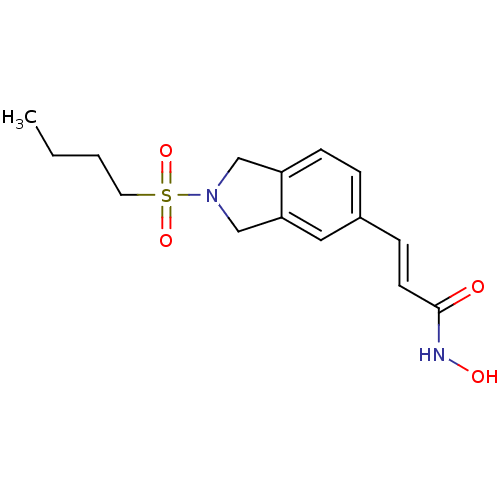
(CHEMBL1808627)Show InChI InChI=1S/C15H20N2O4S/c1-2-3-8-22(20,21)17-10-13-6-4-12(9-14(13)11-17)5-7-15(18)16-19/h4-7,9,19H,2-3,8,10-11H2,1H3,(H,16,18)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349493

(CHEMBL1808633)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C16H15N3O4S/c20-16(18-21)6-4-12-3-5-13-10-19(11-14(13)8-12)24(22,23)15-2-1-7-17-9-15/h1-9,21H,10-11H2,(H,18,20)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24624
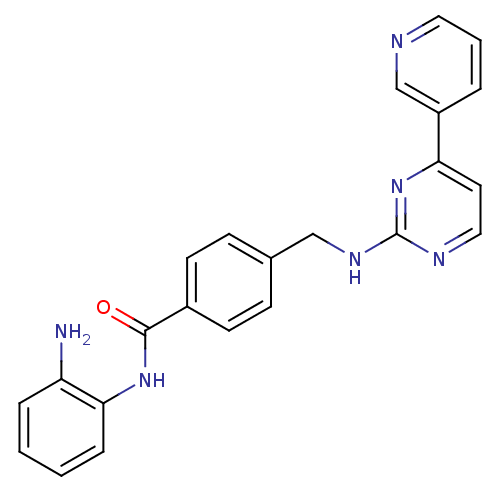
(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349499

(CHEMBL1808639)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C18H15F3N2O4S/c19-18(20,21)15-2-1-3-16(9-15)28(26,27)23-10-13-6-4-12(8-14(13)11-23)5-7-17(24)22-25/h1-9,25H,10-11H2,(H,22,24)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349498

(CHEMBL1808638)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H15F3N2O4S/c19-18(20,21)15-4-6-16(7-5-15)28(26,27)23-10-13-3-1-12(9-14(13)11-23)2-8-17(24)22-25/h1-9,25H,10-11H2,(H,22,24)/b8-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349506

(CHEMBL1808646)Show SMILES Cc1noc(c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1)-c1ccccc1 Show InChI InChI=1S/C23H23N3O3/c1-16-21(23(29-25-16)18-5-3-2-4-6-18)11-12-26-14-19-9-7-17(13-20(19)15-26)8-10-22(27)24-28/h2-10,13,28H,11-12,14-15H2,1H3,(H,24,27)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349490

(CHEMBL1808630)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H16N2O4S/c20-17(18-21)9-7-13-6-8-14-11-19(12-15(14)10-13)24(22,23)16-4-2-1-3-5-16/h1-10,21H,11-12H2,(H,18,20)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349482
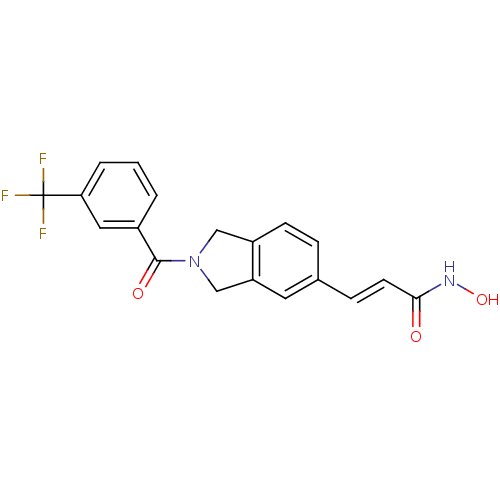
(CHEMBL1808622)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)C(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H15F3N2O3/c20-19(21,22)16-3-1-2-13(9-16)18(26)24-10-14-6-4-12(8-15(14)11-24)5-7-17(25)23-27/h1-9,27H,10-11H2,(H,23,25)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349502

(CHEMBL1808642)Show InChI InChI=1S/C18H18N2O2/c21-18(19-22)9-7-14-6-8-16-12-20(13-17(16)10-14)11-15-4-2-1-3-5-15/h1-10,22H,11-13H2,(H,19,21)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349480
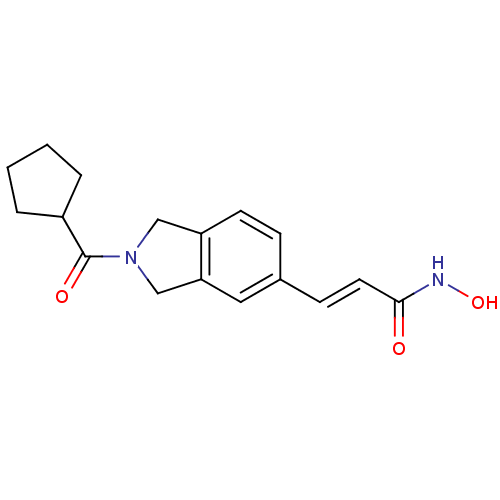
(CHEMBL1808620)Show InChI InChI=1S/C17H20N2O3/c20-16(18-22)8-6-12-5-7-14-10-19(11-15(14)9-12)17(21)13-3-1-2-4-13/h5-9,13,22H,1-4,10-11H2,(H,18,20)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349483

(CHEMBL1808623)Show InChI InChI=1S/C17H15N3O3/c21-16(19-23)6-4-12-3-5-14-10-20(11-15(14)8-12)17(22)13-2-1-7-18-9-13/h1-9,23H,10-11H2,(H,19,21)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349481

(CHEMBL1808621)Show InChI InChI=1S/C18H22N2O3/c21-17(19-23)9-7-13-6-8-15-11-20(12-16(15)10-13)18(22)14-4-2-1-3-5-14/h6-10,14,23H,1-5,11-12H2,(H,19,21)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349479
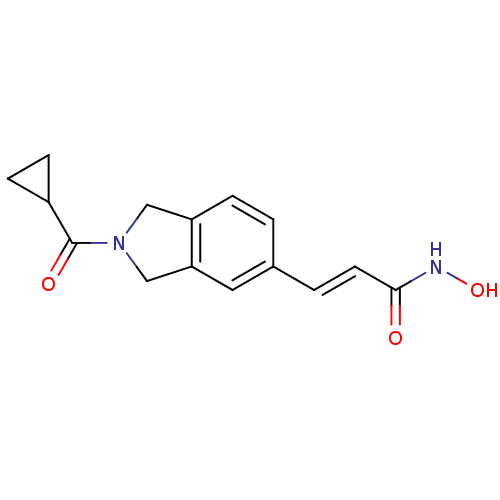
(CHEMBL1808619)Show InChI InChI=1S/C15H16N2O3/c18-14(16-20)6-2-10-1-3-12-8-17(9-13(12)7-10)15(19)11-4-5-11/h1-3,6-7,11,20H,4-5,8-9H2,(H,16,18)/b6-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349478

(CHEMBL1808618)Show InChI InChI=1S/C16H20N2O3/c1-16(2,3)15(20)18-9-12-6-4-11(8-13(12)10-18)5-7-14(19)17-21/h4-8,21H,9-10H2,1-3H3,(H,17,19)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349492
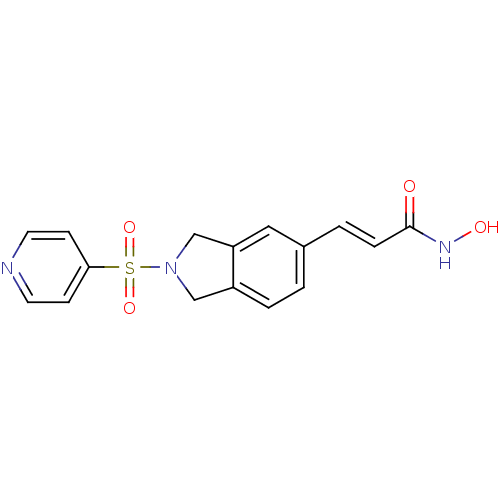
(CHEMBL1808632)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1ccncc1 Show InChI InChI=1S/C16H15N3O4S/c20-16(18-21)4-2-12-1-3-13-10-19(11-14(13)9-12)24(22,23)15-5-7-17-8-6-15/h1-9,21H,10-11H2,(H,18,20)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349477

(CHEMBL1808617)Show InChI InChI=1S/C16H20N2O4/c1-16(2,3)22-15(20)18-9-12-6-4-11(8-13(12)10-18)5-7-14(19)17-21/h4-8,21H,9-10H2,1-3H3,(H,17,19)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM24624

(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349486
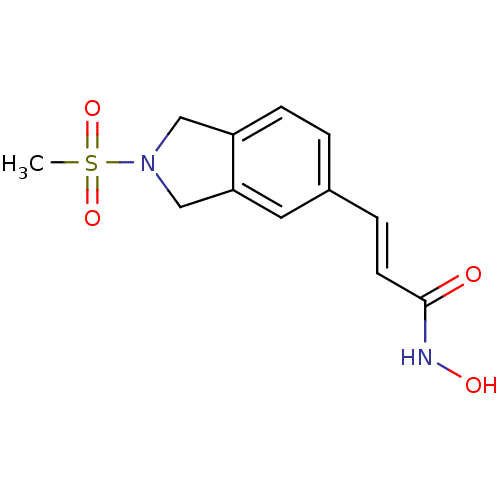
(CHEMBL1808626)Show InChI InChI=1S/C12H14N2O4S/c1-19(17,18)14-7-10-4-2-9(6-11(10)8-14)3-5-12(15)13-16/h2-6,16H,7-8H2,1H3,(H,13,15)/b5-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349488

(CHEMBL1808628)Show InChI InChI=1S/C14H16N2O4S/c17-14(15-18)6-2-10-1-3-11-8-16(9-12(11)7-10)21(19,20)13-4-5-13/h1-3,6-7,13,18H,4-5,8-9H2,(H,15,17)/b6-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314631

(CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H23N3O2/c1-15-19(20-4-2-3-5-21(20)23-15)10-11-25-13-17-8-6-16(12-18(17)14-25)7-9-22(26)24-27/h2-9,12,23,27H,10-11,13-14H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349505

(CHEMBL1808645)Show InChI InChI=1S/C20H20N4O2/c25-20(22-26)7-5-15-4-6-17-13-23(14-18(17)11-15)10-8-16-12-21-24-9-2-1-3-19(16)24/h1-7,9,11-12,26H,8,10,13-14H2,(H,22,25)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314631

(CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H23N3O2/c1-15-19(20-4-2-3-5-21(20)23-15)10-11-25-13-17-8-6-16(12-18(17)14-25)7-9-22(26)24-27/h2-9,12,23,27H,10-11,13-14H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50349504

(CHEMBL1808644)Show SMILES ONC(=O)\C=C\c1ccc2CN(CCc3c[nH]c4ccccc34)Cc2c1 Show InChI InChI=1S/C21H21N3O2/c25-21(23-26)8-6-15-5-7-17-13-24(14-18(17)11-15)10-9-16-12-22-20-4-2-1-3-19(16)20/h1-8,11-12,22,26H,9-10,13-14H2,(H,23,25)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349503

(CHEMBL1808643)Show InChI InChI=1S/C19H26N2O2/c22-19(20-23)9-7-16-6-8-17-13-21(14-18(17)12-16)11-10-15-4-2-1-3-5-15/h6-9,12,15,23H,1-5,10-11,13-14H2,(H,20,22)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349502

(CHEMBL1808642)Show InChI InChI=1S/C18H18N2O2/c21-18(19-22)9-7-14-6-8-16-12-20(13-17(16)10-14)11-15-4-2-1-3-5-15/h1-10,22H,11-13H2,(H,19,21)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349485

(CHEMBL1808625)Show SMILES Cc1[nH]c2ccccc2c1CC(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H21N3O3/c1-14-19(18-4-2-3-5-20(18)23-14)11-22(27)25-12-16-8-6-15(10-17(16)13-25)7-9-21(26)24-28/h2-10,23,28H,11-13H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG by radioligand binding assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349478

(CHEMBL1808618)Show InChI InChI=1S/C16H20N2O3/c1-16(2,3)15(20)18-9-12-6-4-11(8-13(12)10-18)5-7-14(19)17-21/h4-8,21H,9-10H2,1-3H3,(H,17,19)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349481

(CHEMBL1808621)Show InChI InChI=1S/C18H22N2O3/c21-17(19-23)9-7-13-6-8-15-11-20(12-16(15)10-13)18(22)14-4-2-1-3-5-14/h6-10,14,23H,1-5,11-12H2,(H,19,21)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349482

(CHEMBL1808622)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)C(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H15F3N2O3/c20-19(21,22)16-3-1-2-13(9-16)18(26)24-10-14-6-4-12(8-15(14)11-24)5-7-17(25)23-27/h1-9,27H,10-11H2,(H,23,25)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349487

(CHEMBL1808627)Show InChI InChI=1S/C15H20N2O4S/c1-2-3-8-22(20,21)17-10-13-6-4-12(9-14(13)11-17)5-7-15(18)16-19/h4-7,9,19H,2-3,8,10-11H2,1H3,(H,16,18)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349504

(CHEMBL1808644)Show SMILES ONC(=O)\C=C\c1ccc2CN(CCc3c[nH]c4ccccc34)Cc2c1 Show InChI InChI=1S/C21H21N3O2/c25-21(23-26)8-6-15-5-7-17-13-24(14-18(17)11-15)10-9-16-12-22-20-4-2-1-3-19(16)20/h1-8,11-12,22,26H,9-10,13-14H2,(H,23,25)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349485

(CHEMBL1808625)Show SMILES Cc1[nH]c2ccccc2c1CC(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H21N3O3/c1-14-19(18-4-2-3-5-20(18)23-14)11-22(27)25-12-16-8-6-15(10-17(16)13-25)7-9-21(26)24-28/h2-10,23,28H,11-13H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349491

(CHEMBL1808631)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C15H14N2O4S2/c18-14(16-19)6-4-11-3-5-12-9-17(10-13(12)8-11)23(20,21)15-2-1-7-22-15/h1-8,19H,9-10H2,(H,16,18)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349494

(CHEMBL1808634)Show SMILES Cn1cnc(c1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C15H16N4O4S/c1-18-9-15(16-10-18)24(22,23)19-7-12-4-2-11(6-13(12)8-19)3-5-14(20)17-21/h2-6,9-10,21H,7-8H2,1H3,(H,17,20)/b5-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349496

(CHEMBL1808636)Show SMILES Cc1cccc(c1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O4S/c1-13-3-2-4-17(9-13)25(23,24)20-11-15-7-5-14(10-16(15)12-20)6-8-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349506

(CHEMBL1808646)Show SMILES Cc1noc(c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1)-c1ccccc1 Show InChI InChI=1S/C23H23N3O3/c1-16-21(23(29-25-16)18-5-3-2-4-6-18)11-12-26-14-19-9-7-17(13-20(19)15-26)8-10-22(27)24-28/h2-10,13,28H,11-12,14-15H2,1H3,(H,24,27)/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349480

(CHEMBL1808620)Show InChI InChI=1S/C17H20N2O3/c20-16(18-22)8-6-12-5-7-14-10-19(11-15(14)9-12)17(21)13-3-1-2-4-13/h5-9,13,22H,1-4,10-11H2,(H,18,20)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349484

(CHEMBL1808624)Show InChI InChI=1S/C19H18N2O3/c22-18(20-24)9-7-15-6-8-16-12-21(13-17(16)10-15)19(23)11-14-4-2-1-3-5-14/h1-10,24H,11-13H2,(H,20,22)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349490

(CHEMBL1808630)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H16N2O4S/c20-17(18-21)9-7-13-6-8-14-11-19(12-15(14)10-13)24(22,23)16-4-2-1-3-5-16/h1-10,21H,11-12H2,(H,18,20)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349495

(CHEMBL1808635)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O4S/c1-13-2-7-17(8-3-13)25(23,24)20-11-15-6-4-14(10-16(15)12-20)5-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349497

(CHEMBL1808637)Show SMILES Cc1ccccc1S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O4S/c1-13-4-2-3-5-17(13)25(23,24)20-11-15-8-6-14(10-16(15)12-20)7-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349479

(CHEMBL1808619)Show InChI InChI=1S/C15H16N2O3/c18-14(16-20)6-2-10-1-3-12-8-17(9-13(12)7-10)15(19)11-4-5-11/h1-3,6-7,11,20H,4-5,8-9H2,(H,16,18)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349486

(CHEMBL1808626)Show InChI InChI=1S/C12H14N2O4S/c1-19(17,18)14-7-10-4-2-9(6-11(10)8-14)3-5-12(15)13-16/h2-6,16H,7-8H2,1H3,(H,13,15)/b5-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349489

(CHEMBL1808629)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C18H18N2O4S/c21-18(19-22)9-7-14-6-8-16-11-20(12-17(16)10-14)25(23,24)13-15-4-2-1-3-5-15/h1-10,22H,11-13H2,(H,19,21)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349492

(CHEMBL1808632)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1ccncc1 Show InChI InChI=1S/C16H15N3O4S/c20-16(18-21)4-2-12-1-3-13-10-19(11-14(13)9-12)24(22,23)15-5-7-17-8-6-15/h1-9,21H,10-11H2,(H,18,20)/b4-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349483

(CHEMBL1808623)Show InChI InChI=1S/C17H15N3O3/c21-16(19-23)6-4-12-3-5-14-10-20(11-15(14)8-12)17(22)13-2-1-7-18-9-13/h1-9,23H,10-11H2,(H,19,21)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349488

(CHEMBL1808628)Show InChI InChI=1S/C14H16N2O4S/c17-14(15-18)6-2-10-1-3-11-8-16(9-12(11)7-10)21(19,20)13-4-5-13/h1-3,6-7,13,18H,4-5,8-9H2,(H,15,17)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349493

(CHEMBL1808633)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C16H15N3O4S/c20-16(18-21)6-4-12-3-5-13-10-19(11-14(13)8-12)24(22,23)15-2-1-7-17-9-15/h1-9,21H,10-11H2,(H,18,20)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24624

(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349477

(CHEMBL1808617)Show InChI InChI=1S/C16H20N2O4/c1-16(2,3)22-15(20)18-9-12-6-4-11(8-13(12)10-18)5-7-14(19)17-21/h4-8,21H,9-10H2,1-3H3,(H,17,19)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349500

(CHEMBL1808640)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O5S/c1-25-16-5-7-17(8-6-16)26(23,24)20-11-14-4-2-13(10-15(14)12-20)3-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349498

(CHEMBL1808638)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H15F3N2O4S/c19-18(20,21)15-4-6-16(7-5-15)28(26,27)23-10-13-3-1-12(9-14(13)11-23)2-8-17(24)22-25/h1-9,25H,10-11H2,(H,22,24)/b8-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349499

(CHEMBL1808639)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C18H15F3N2O4S/c19-18(20,21)15-2-1-3-16(9-15)28(26,27)23-10-13-6-4-12(8-14(13)11-23)5-7-17(24)22-25/h1-9,25H,10-11H2,(H,22,24)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50349501

(CHEMBL1808641)Show SMILES COc1cccc(c1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O5S/c1-25-16-3-2-4-17(10-16)26(23,24)20-11-14-7-5-13(9-15(14)12-20)6-8-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp assay |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50349484

(CHEMBL1808624)Show InChI InChI=1S/C19H18N2O3/c22-18(20-24)9-7-15-6-8-16-12-21(13-17(16)10-15)19(23)11-14-4-2-1-3-5-14/h1-10,24H,11-13H2,(H,20,22)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50349491

(CHEMBL1808631)Show SMILES ONC(=O)\C=C\c1ccc2CN(Cc2c1)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C15H14N2O4S2/c18-14(16-19)6-4-11-3-5-12-9-17(10-13(12)8-11)23(20,21)15-2-1-7-22-15/h1-8,19H,9-10H2,(H,16,18)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50349505

(CHEMBL1808645)Show InChI InChI=1S/C20H20N4O2/c25-20(22-26)7-5-15-4-6-17-13-23(14-18(17)11-15)10-8-16-12-21-24-9-2-1-3-19(16)24/h1-7,9,11-12,26H,8,10,13-14H2,(H,22,25)/b7-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50349500

(CHEMBL1808640)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C18H18N2O5S/c1-25-16-5-7-17(8-6-16)26(23,24)20-11-14-4-2-13(10-15(14)12-20)3-9-18(21)19-22/h2-10,22H,11-12H2,1H3,(H,19,21)/b9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50349503

(CHEMBL1808643)Show InChI InChI=1S/C19H26N2O2/c22-19(20-23)9-7-16-6-8-17-13-21(14-18(17)12-16)11-10-15-4-2-1-3-5-15/h6-9,12,15,23H,1-5,10-11,13-14H2,(H,20,22)/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data