 Found 40 hits of Enzyme Inhibition Constant Data
Found 40 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50018849

(4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...)Show SMILES OC(=O)[C@@H]1C[C@H](CN1C(=O)CP(O)(=O)CCCCc1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H34NO5P/c25-22(17-30(28,29)14-8-7-11-18-9-3-1-4-10-18)24-16-20(15-21(24)23(26)27)19-12-5-2-6-13-19/h1,3-4,9-10,19-21H,2,5-8,11-17H2,(H,26,27)(H,28,29)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50393210
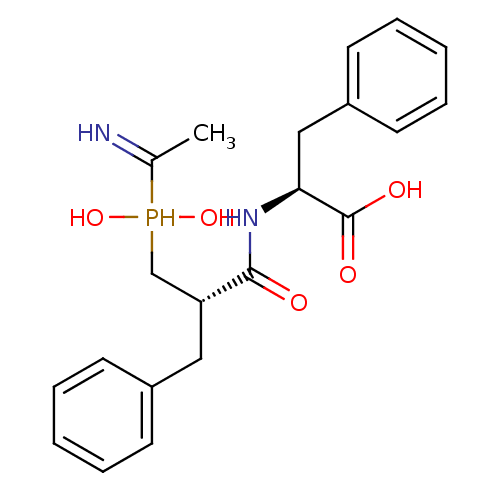
(CHEMBL1235787)Show SMILES CC(=N)P(O)(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C21H27N2O5P/c1-15(22)29(27,28)14-18(12-16-8-4-2-5-9-16)20(24)23-19(21(25)26)13-17-10-6-3-7-11-17/h2-11,18-19,22,27-29H,12-14H2,1H3,(H,23,24)(H,25,26)/t18-,19+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50393211
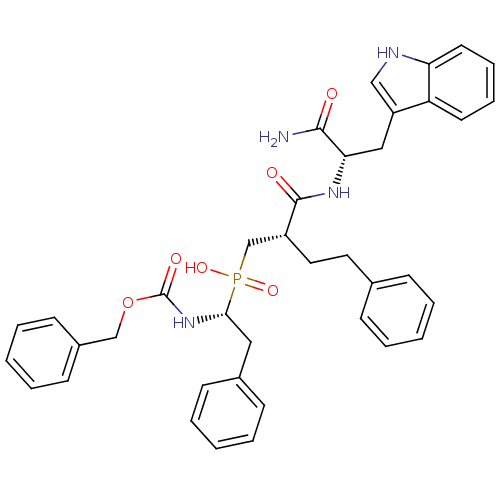
(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-8 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303327
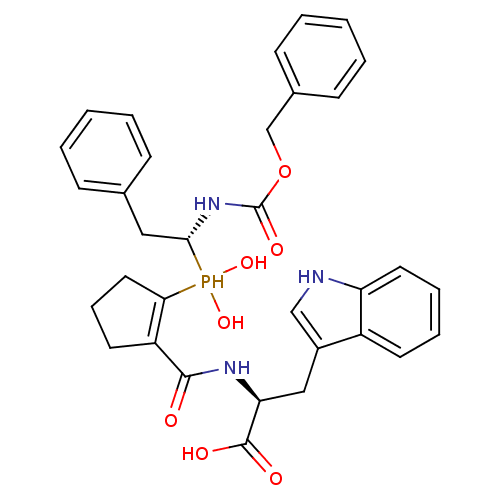
((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1=C(CCC1)P(O)(O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,t:19| Show InChI InChI=1S/C33H36N3O7P/c37-31(35-28(32(38)39)19-24-20-34-27-16-8-7-14-25(24)27)26-15-9-17-29(26)44(41,42)30(18-22-10-3-1-4-11-22)36-33(40)43-21-23-12-5-2-6-13-23/h1-8,10-14,16,20,28,30,34,41-42,44H,9,15,17-19,21H2,(H,35,37)(H,36,40)(H,38,39)/t28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-3
(Homo sapiens (Human)) | BDBM50393211

(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-11 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50393213
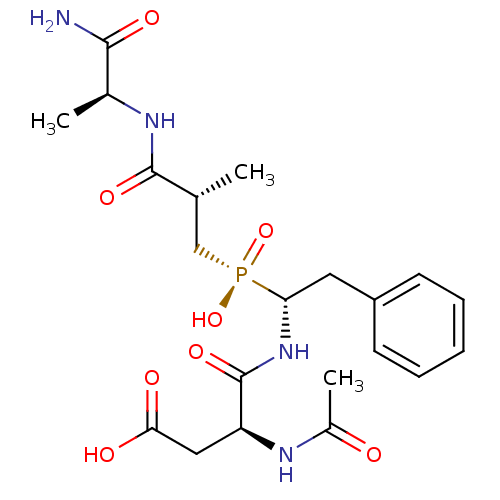
(CHEMBL1235767)Show SMILES C[C@H](C[P@](O)(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C21H31N4O8P/c1-12(20(30)23-13(2)19(22)29)11-34(32,33)17(9-15-7-5-4-6-8-15)25-21(31)16(10-18(27)28)24-14(3)26/h4-8,12-13,16-17H,9-11H2,1-3H3,(H2,22,29)(H,23,30)(H,24,26)(H,25,31)(H,27,28)(H,32,33)/t12-,13+,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50393211

(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Leishmania major) | BDBM50098390
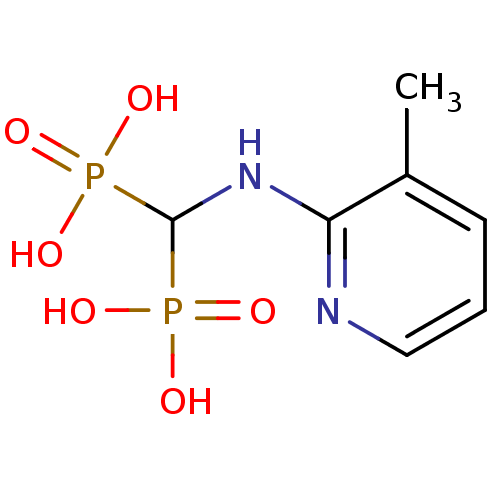
((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major FPPS |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50393211

(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-2 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50393212

(CHEMBL2153739)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)c1cc(no1)-c1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C45H42N5O8P/c46-43(51)40(25-33-27-47-38-22-11-10-21-36(33)38)48-44(52)37(41-26-39(50-58-41)32-17-12-20-35(24-32)57-34-18-8-3-9-19-34)29-59(54,55)42(23-30-13-4-1-5-14-30)49-45(53)56-28-31-15-6-2-7-16-31/h1-22,24,26-27,37,40,42,47H,23,25,28-29H2,(H2,46,51)(H,48,52)(H,49,53)(H,54,55)/t37-,40-,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-13 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50129683
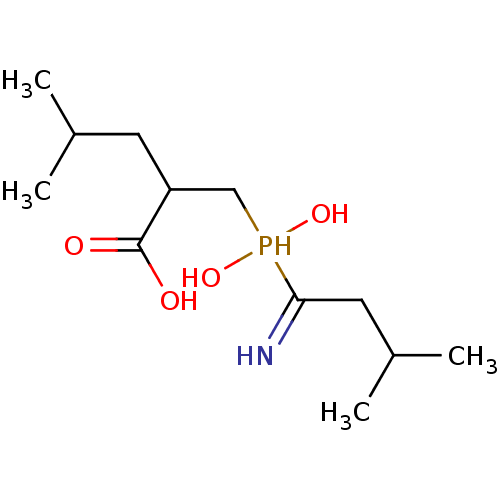
(2-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoylmet...)Show InChI InChI=1S/C12H26NO4P/c1-8(2)5-10(12(14)15)7-18(16,17)11(13)6-9(3)4/h8-10,13,16-18H,5-7H2,1-4H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of LAP |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393211

(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50393207
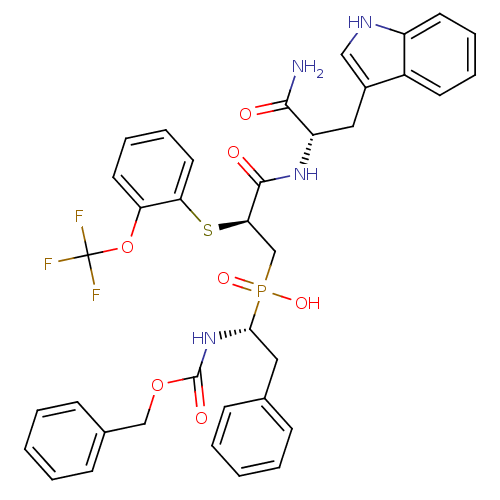
(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-1 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Stromelysin-3
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-11 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50129683

(2-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoylmet...)Show InChI InChI=1S/C12H26NO4P/c1-8(2)5-10(12(14)15)7-18(16,17)11(13)6-9(3)4/h8-10,13,16-18H,5-7H2,1-4H3,(H,14,15) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50393211

(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50393211

(CHEMBL2153737)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H41N4O6P/c39-36(43)34(23-31-24-40-33-19-11-10-18-32(31)33)41-37(44)30(21-20-27-12-4-1-5-13-27)26-49(46,47)35(22-28-14-6-2-7-15-28)42-38(45)48-25-29-16-8-3-9-17-29/h1-19,24,30,34-35,40H,20-23,25-26H2,(H2,39,43)(H,41,44)(H,42,45)(H,46,47)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-1 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393212

(CHEMBL2153739)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)c1cc(no1)-c1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C45H42N5O8P/c46-43(51)40(25-33-27-47-38-22-11-10-21-36(33)38)48-44(52)37(41-26-39(50-58-41)32-17-12-20-35(24-32)57-34-18-8-3-9-19-34)29-59(54,55)42(23-30-13-4-1-5-14-30)49-45(53)56-28-31-15-6-2-7-16-31/h1-22,24,26-27,37,40,42,47H,23,25,28-29H2,(H2,46,51)(H,48,52)(H,49,53)(H,54,55)/t37-,40-,42+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50393213

(CHEMBL1235767)Show SMILES C[C@H](C[P@](O)(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C21H31N4O8P/c1-12(20(30)23-13(2)19(22)29)11-34(32,33)17(9-15-7-5-4-6-8-15)25-21(31)16(10-18(27)28)24-14(3)26/h4-8,12-13,16-17H,9-11H2,1-3H3,(H2,22,29)(H,23,30)(H,24,26)(H,25,31)(H,27,28)(H,32,33)/t12-,13+,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303327

((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1=C(CCC1)P(O)(O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,t:19| Show InChI InChI=1S/C33H36N3O7P/c37-31(35-28(32(38)39)19-24-20-34-27-16-8-7-14-25(24)27)26-15-9-17-29(26)44(41,42)30(18-22-10-3-1-4-11-22)36-33(40)43-21-23-12-5-2-6-13-23/h1-8,10-14,16,20,28,30,34,41-42,44H,9,15,17-19,21H2,(H,35,37)(H,36,40)(H,38,39)/t28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50393208
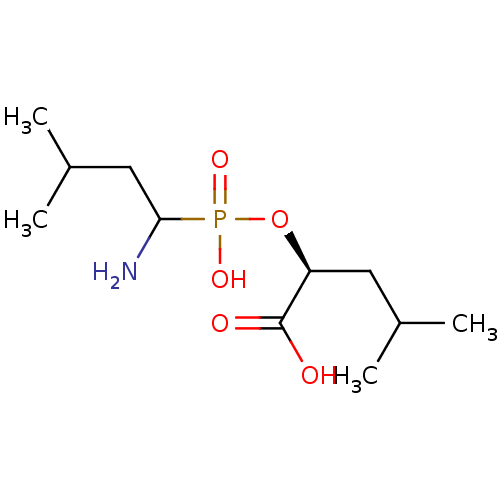
(CHEMBL2153735)Show SMILES CC(C)CC(N)P(O)(=O)O[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C11H24NO5P/c1-7(2)5-9(11(13)14)17-18(15,16)10(12)6-8(3)4/h7-10H,5-6,12H2,1-4H3,(H,13,14)(H,15,16)/t9-,10?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of LAP |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-2 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-13 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50393207

(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-8 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50393206
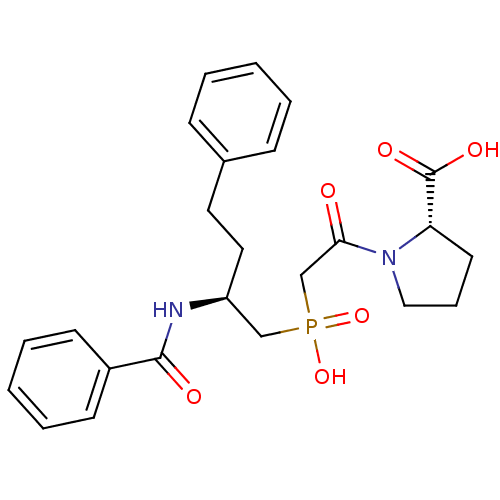
(CHEMBL2153745)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CP(O)(=O)C[C@H](CCc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C24H29N2O6P/c27-22(26-15-7-12-21(26)24(29)30)17-33(31,32)16-20(14-13-18-8-3-1-4-9-18)25-23(28)19-10-5-2-6-11-19/h1-6,8-11,20-21H,7,12-17H2,(H,25,28)(H,29,30)(H,31,32)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50393204
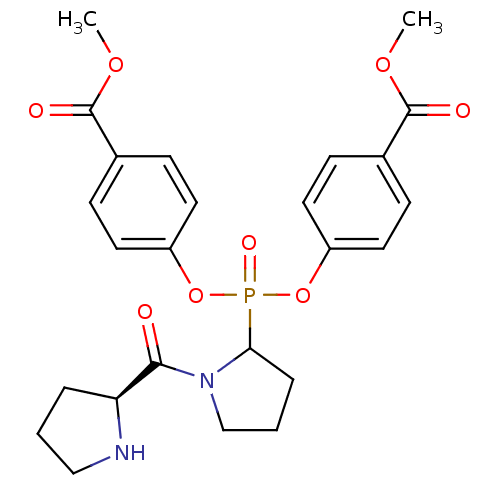
(CHEMBL2153743)Show SMILES COC(=O)c1ccc(OP(=O)(Oc2ccc(cc2)C(=O)OC)C2CCCN2C(=O)[C@@H]2CCCN2)cc1 |r| Show InChI InChI=1S/C25H29N2O8P/c1-32-24(29)17-7-11-19(12-8-17)34-36(31,35-20-13-9-18(10-14-20)25(30)33-2)22-6-4-16-27(22)23(28)21-5-3-15-26-21/h7-14,21-22,26H,3-6,15-16H2,1-2H3/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50393205
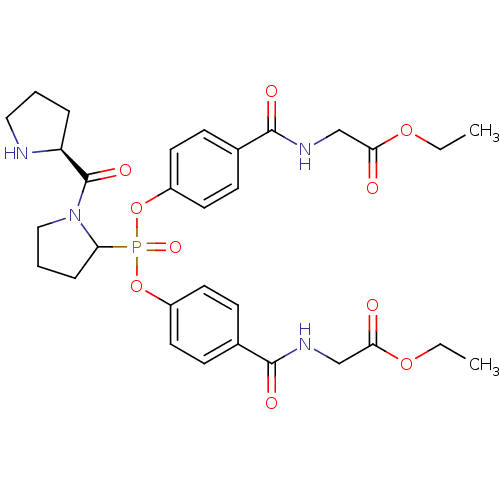
(CHEMBL2153744)Show SMILES CCOC(=O)CNC(=O)c1ccc(OP(=O)(Oc2ccc(cc2)C(=O)NCC(=O)OCC)C2CCCN2C(=O)[C@@H]2CCCN2)cc1 |r| Show InChI InChI=1S/C31H39N4O10P/c1-3-42-27(36)19-33-29(38)21-9-13-23(14-10-21)44-46(41,26-8-6-18-35(26)31(40)25-7-5-17-32-25)45-24-15-11-22(12-16-24)30(39)34-20-28(37)43-4-2/h9-16,25-26,32H,3-8,17-20H2,1-2H3,(H,33,38)(H,34,39)/t25-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135821
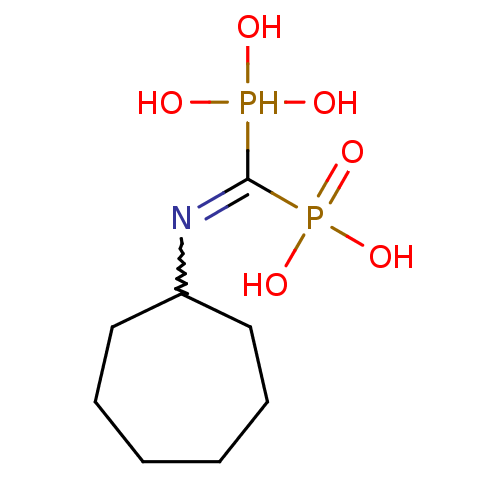
((Bis-phosphono-methyl)-cycloheptyl-ammonium | (Cyc...)Show InChI InChI=1S/C8H19NO6P2/c10-16(11,12)8(17(13,14)15)9-7-5-3-1-2-4-6-7/h7,10-12,16H,1-6H2,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25313

((4-amino-1-hydroxy-1-phosphonobutyl)phosphonic aci...)Show InChI InChI=1S/C4H13NO7P2/c5-3-1-2-4(6,13(7,8)9)14(10,11)12/h6H,1-3,5H2,(H2,7,8,9)(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12581
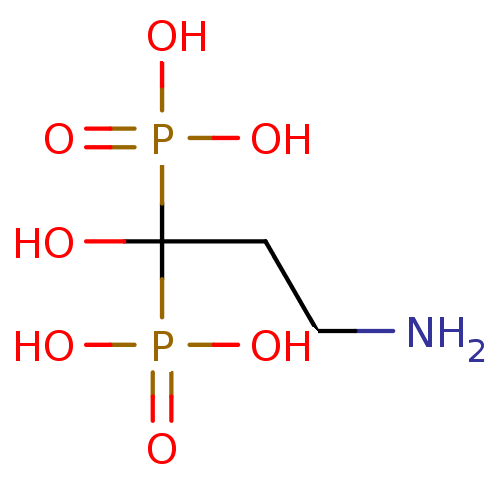
((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50393203

(CHEMBL2153742)Show SMILES CC(=O)Nc1ccc(OP(=O)(Oc2ccc(NC(C)=O)cc2)C2CCCN2C(=O)[C@@H]2CCCN2)cc1 |r| Show InChI InChI=1S/C25H31N4O6P/c1-17(30)27-19-7-11-21(12-8-19)34-36(33,35-22-13-9-20(10-14-22)28-18(2)31)24-6-4-16-29(24)25(32)23-5-3-15-26-23/h7-14,23-24,26H,3-6,15-16H2,1-2H3,(H,27,30)(H,28,31)/t23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50393202

(CHEMBL1182947)Show SMILES O=C([C@@H]1CCCN1)N1CCCC1P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C21H25N2O4P/c24-21(19-13-7-15-22-19)23-16-8-14-20(23)28(25,26-17-9-3-1-4-10-17)27-18-11-5-2-6-12-18/h1-6,9-12,19-20,22H,7-8,13-16H2/t19-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data