

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxa40 (Acinetobacter baumannii) | BDBM92463 (Imipenem) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50140672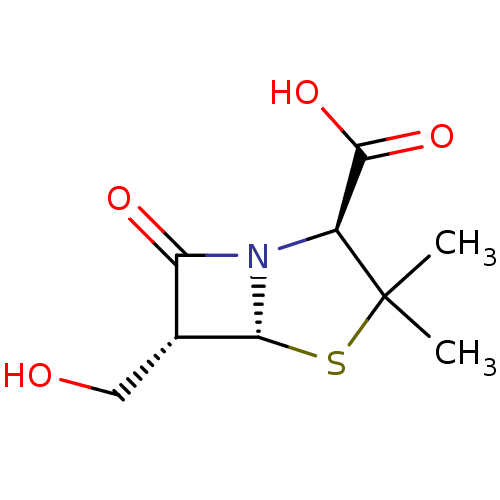 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92461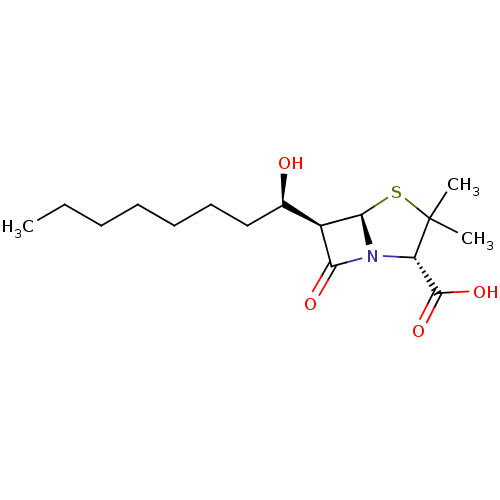 (Beta-lactam compound, 4) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92464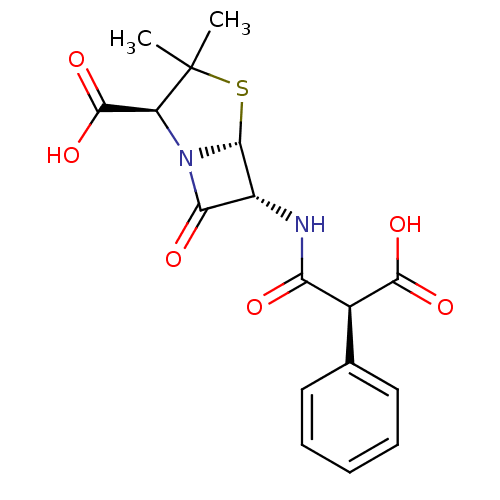 (Carbenicillin) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50140671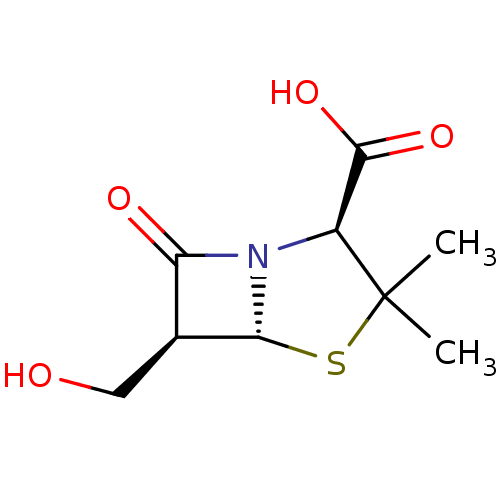 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92462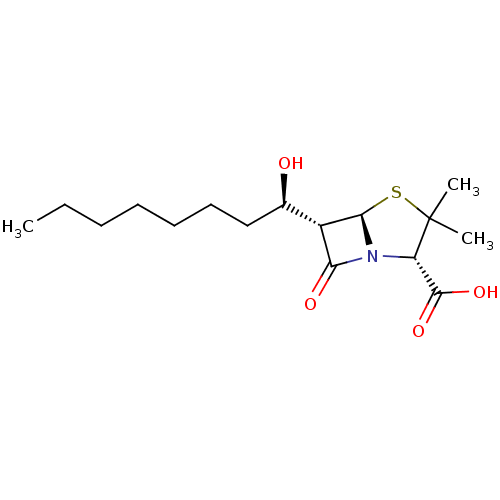 (Beta-lactam compound, 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||