

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355311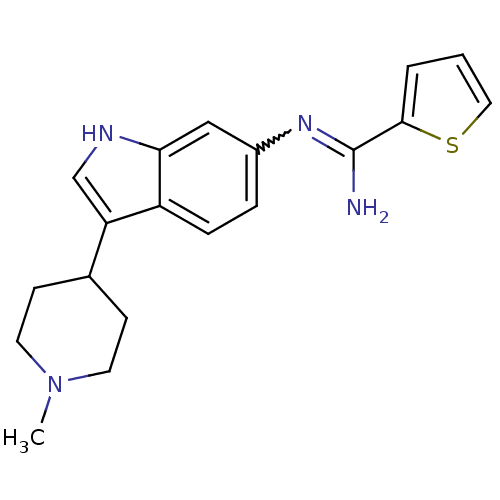 (CHEMBL1835114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355309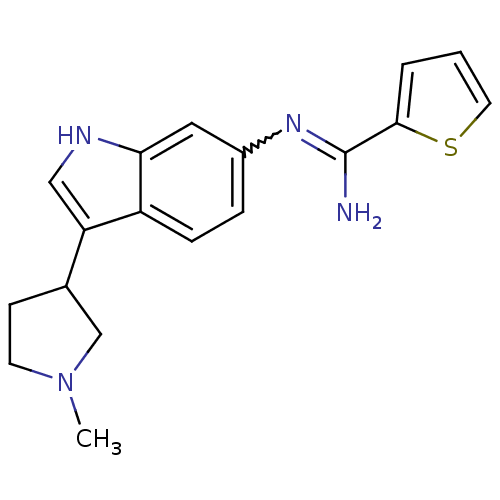 (CHEMBL1835013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106736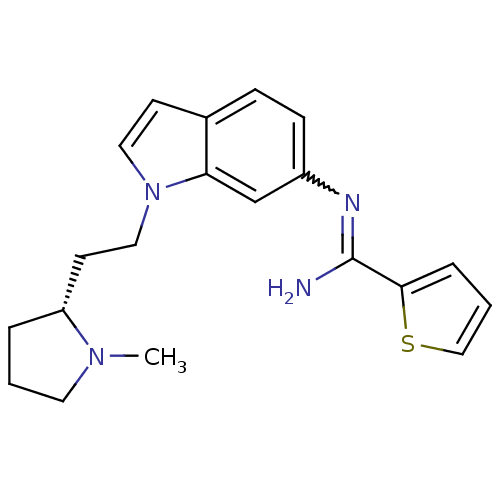 (CHEMBL1823365 | US8586620, 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355313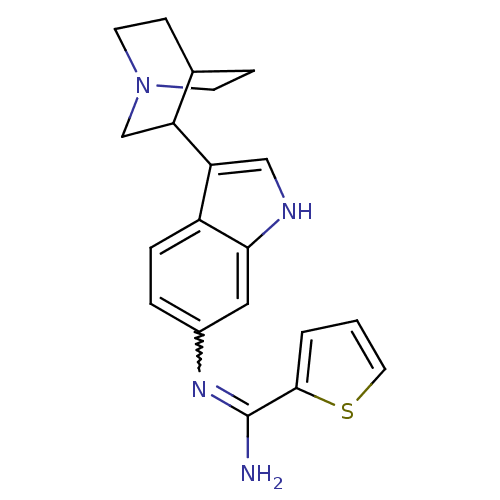 (CHEMBL1835116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM92900 (CHEMBL1835117 | L-Arginine, 2 | NMMA, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50098937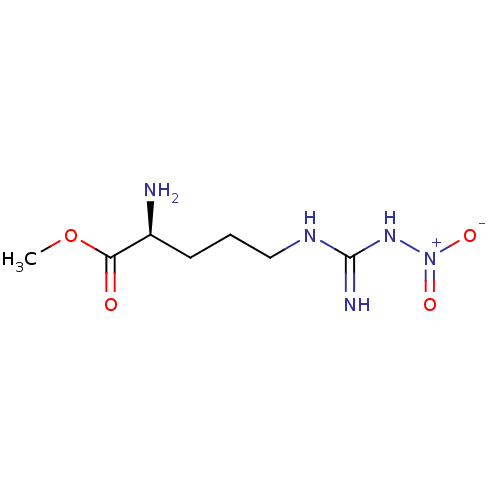 ((S)-methyl 2-amino-5-(amino(nitroamino)methyleneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50098937 ((S)-methyl 2-amino-5-(amino(nitroamino)methyleneam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50098937 ((S)-methyl 2-amino-5-(amino(nitroamino)methyleneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM92900 (CHEMBL1835117 | L-Arginine, 2 | NMMA, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355312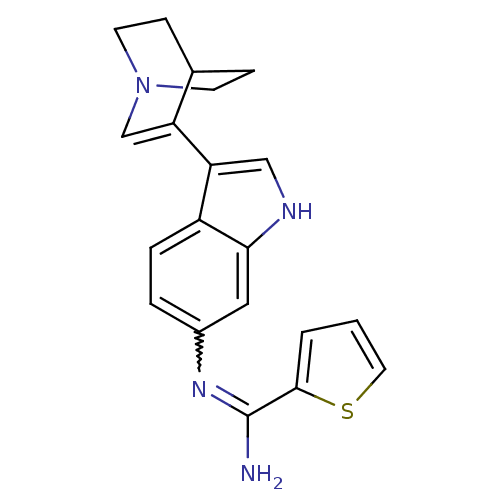 (CHEMBL1835115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM92900 (CHEMBL1835117 | L-Arginine, 2 | NMMA, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355309 (CHEMBL1835013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355309 (CHEMBL1835013) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355312 (CHEMBL1835115) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355311 (CHEMBL1835114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355311 (CHEMBL1835114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 using CEC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50355312 (CHEMBL1835115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 using CEC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 using AMMC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 using MFC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using BFC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using BFC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 using CEC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 using AMMC as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50355310 (CHEMBL1835113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using BQ as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using BQ as substrate | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50355313 (CHEMBL1835116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||