 Found 39 hits of Enzyme Inhibition Constant Data
Found 39 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357272
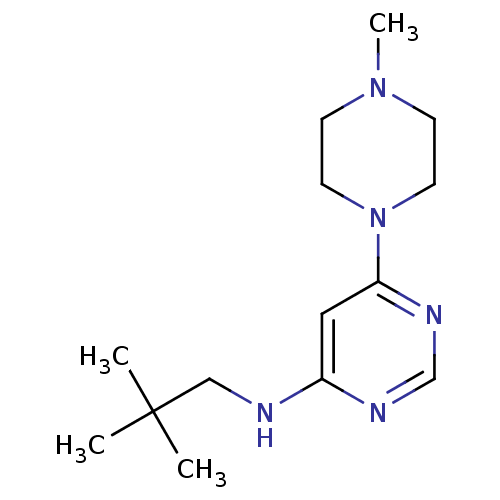
(CHEMBL1916498)Show InChI InChI=1S/C14H25N5/c1-14(2,3)10-15-12-9-13(17-11-16-12)19-7-5-18(4)6-8-19/h9,11H,5-8,10H2,1-4H3,(H,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357277
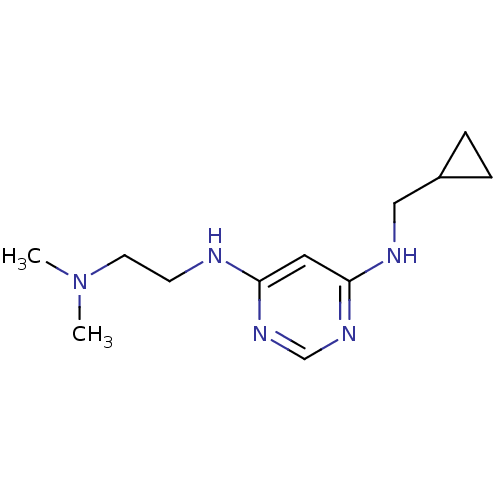
(CHEMBL1916505)Show InChI InChI=1S/C12H21N5/c1-17(2)6-5-13-11-7-12(16-9-15-11)14-8-10-3-4-10/h7,9-10H,3-6,8H2,1-2H3,(H2,13,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50179335
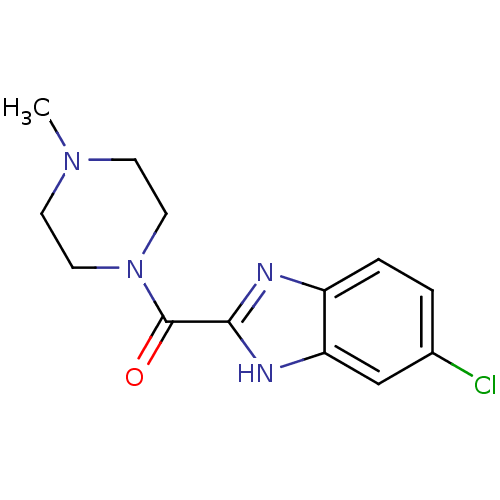
((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...)Show InChI InChI=1S/C13H15ClN4O/c1-17-4-6-18(7-5-17)13(19)12-15-10-3-2-9(14)8-11(10)16-12/h2-3,8H,4-7H2,1H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50179335

((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...)Show InChI InChI=1S/C13H15ClN4O/c1-17-4-6-18(7-5-17)13(19)12-15-10-3-2-9(14)8-11(10)16-12/h2-3,8H,4-7H2,1H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357273
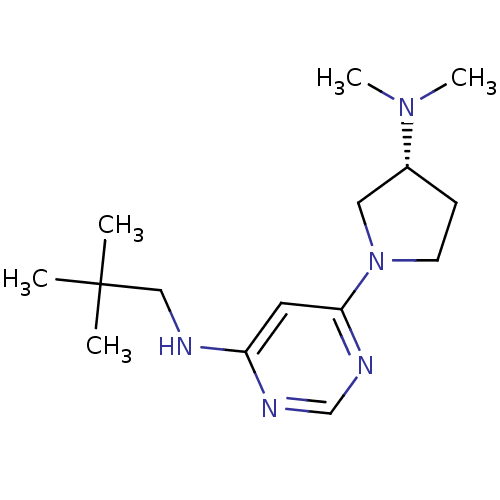
(CHEMBL1916499)Show SMILES CN(C)[C@@H]1CCN(C1)c1cc(NCC(C)(C)C)ncn1 |r| Show InChI InChI=1S/C15H27N5/c1-15(2,3)10-16-13-8-14(18-11-17-13)20-7-6-12(9-20)19(4)5/h8,11-12H,6-7,9-10H2,1-5H3,(H,16,17,18)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357273

(CHEMBL1916499)Show SMILES CN(C)[C@@H]1CCN(C1)c1cc(NCC(C)(C)C)ncn1 |r| Show InChI InChI=1S/C15H27N5/c1-15(2,3)10-16-13-8-14(18-11-17-13)20-7-6-12(9-20)19(4)5/h8,11-12H,6-7,9-10H2,1-5H3,(H,16,17,18)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357274
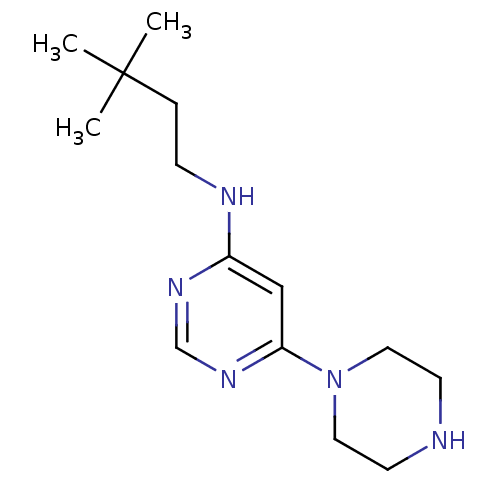
(CHEMBL1916500)Show InChI InChI=1S/C14H25N5/c1-14(2,3)4-5-16-12-10-13(18-11-17-12)19-8-6-15-7-9-19/h10-11,15H,4-9H2,1-3H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50304507
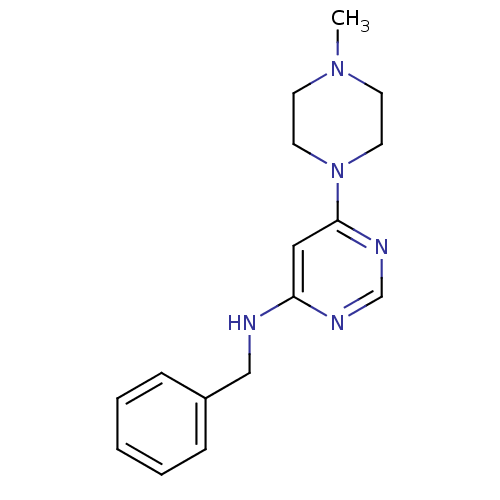
(CHEMBL596562 | N-Benzyl-6-(4-methylpiperazin-1-yl)...)Show InChI InChI=1S/C16H21N5/c1-20-7-9-21(10-8-20)16-11-15(18-13-19-16)17-12-14-5-3-2-4-6-14/h2-6,11,13H,7-10,12H2,1H3,(H,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356890
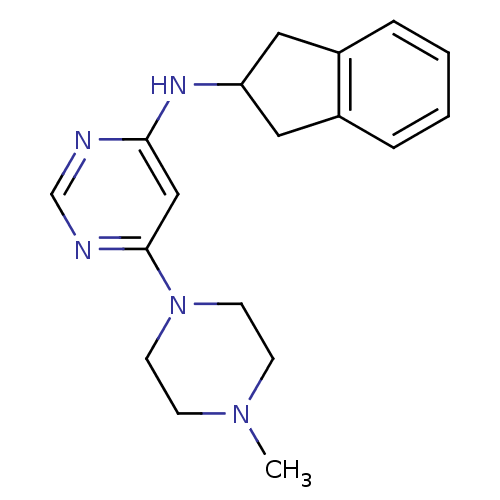
(CHEMBL1915533)Show InChI InChI=1S/C18H23N5/c1-22-6-8-23(9-7-22)18-12-17(19-13-20-18)21-16-10-14-4-2-3-5-15(14)11-16/h2-5,12-13,16H,6-11H2,1H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357275
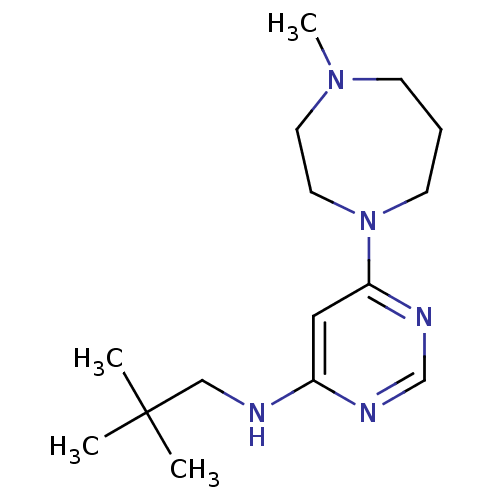
(CHEMBL1916501)Show InChI InChI=1S/C15H27N5/c1-15(2,3)11-16-13-10-14(18-12-17-13)20-7-5-6-19(4)8-9-20/h10,12H,5-9,11H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356878
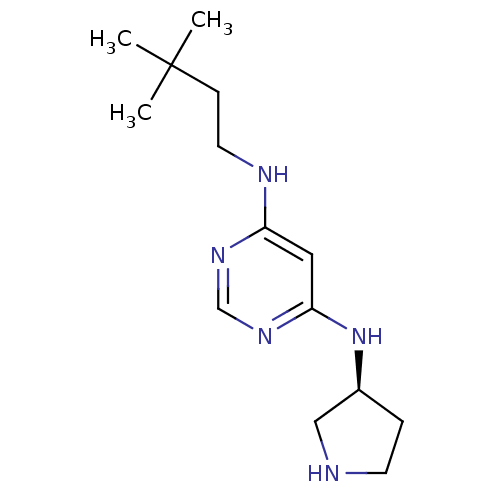
(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357271
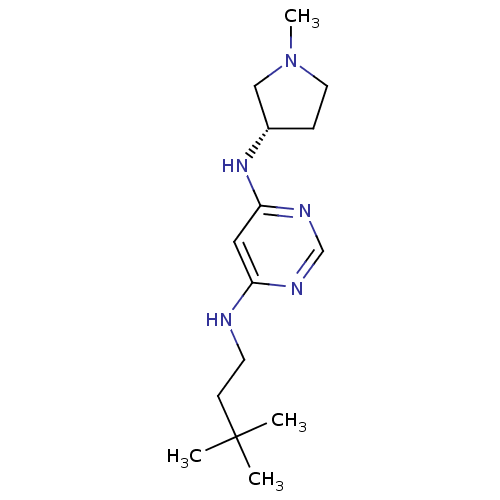
(CHEMBL1916497)Show InChI InChI=1S/C15H27N5/c1-15(2,3)6-7-16-13-9-14(18-11-17-13)19-12-5-8-20(4)10-12/h9,11-12H,5-8,10H2,1-4H3,(H2,16,17,18,19)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 593 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357276
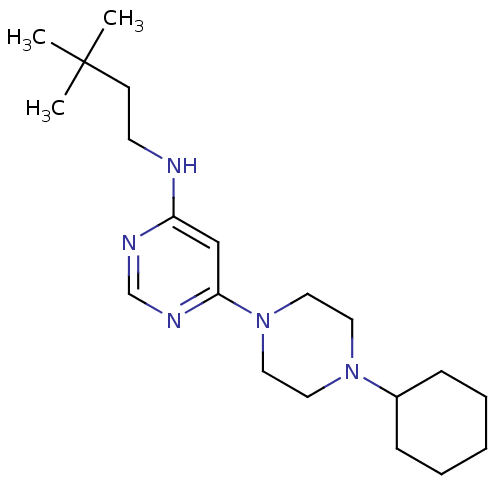
(CHEMBL1916502)Show InChI InChI=1S/C20H35N5/c1-20(2,3)9-10-21-18-15-19(23-16-22-18)25-13-11-24(12-14-25)17-7-5-4-6-8-17/h15-17H,4-14H2,1-3H3,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 601 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357272

(CHEMBL1916498)Show InChI InChI=1S/C14H25N5/c1-14(2,3)10-15-12-9-13(17-11-16-12)19-7-5-18(4)6-8-19/h9,11H,5-8,10H2,1-4H3,(H,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 825 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357278
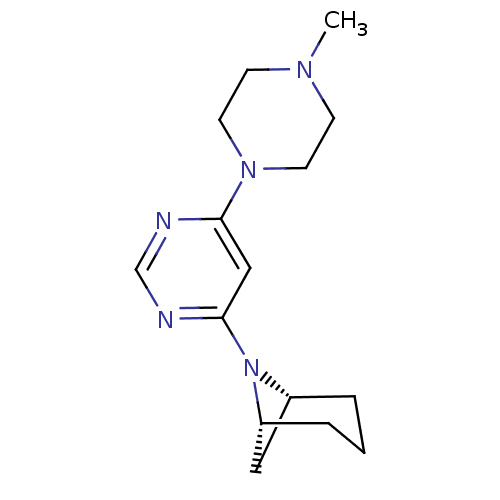
(CHEMBL1916503)Show SMILES CN1CCN(CC1)c1cc(ncn1)N1[C@H]2C[C@H]1CCC2 |r| Show InChI InChI=1S/C15H23N5/c1-18-5-7-19(8-6-18)14-10-15(17-11-16-14)20-12-3-2-4-13(20)9-12/h10-13H,2-9H2,1H3/t12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357279
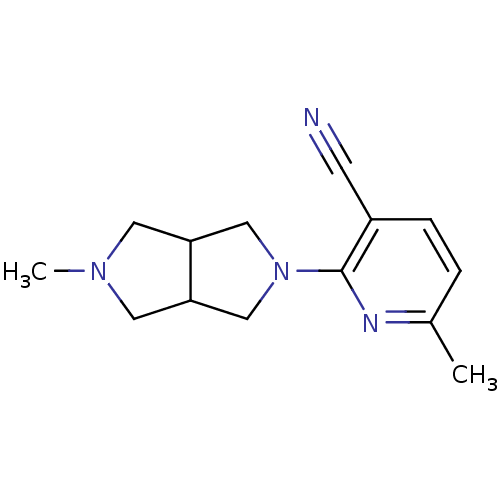
(CHEMBL1916504)Show InChI InChI=1S/C14H18N4/c1-10-3-4-11(5-15)14(16-10)18-8-12-6-17(2)7-13(12)9-18/h3-4,12-13H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357268
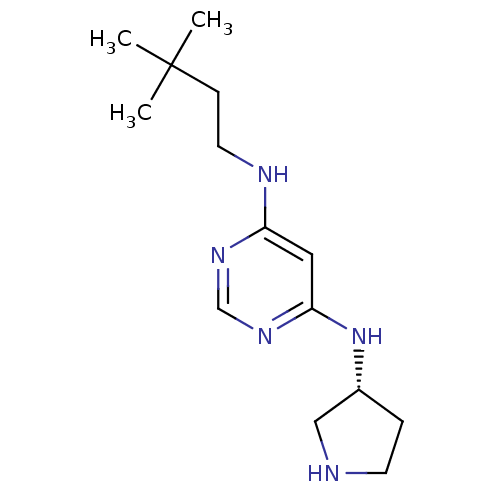
(CHEMBL1916494)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 939 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50060682
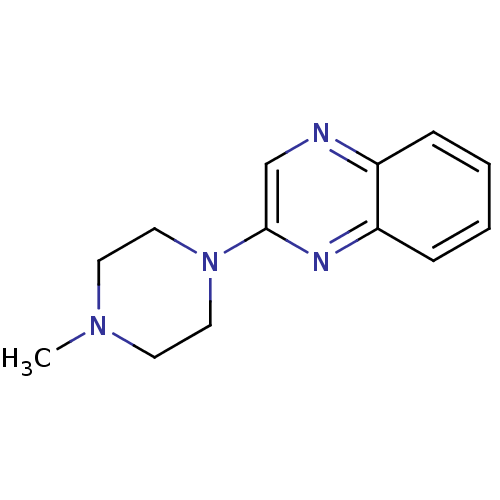
(2-(4-Methyl-piperazin-1-yl)-quinoxaline | CHEMBL12...)Show InChI InChI=1S/C13H16N4/c1-16-6-8-17(9-7-16)13-10-14-11-4-2-3-5-12(11)15-13/h2-5,10H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357267
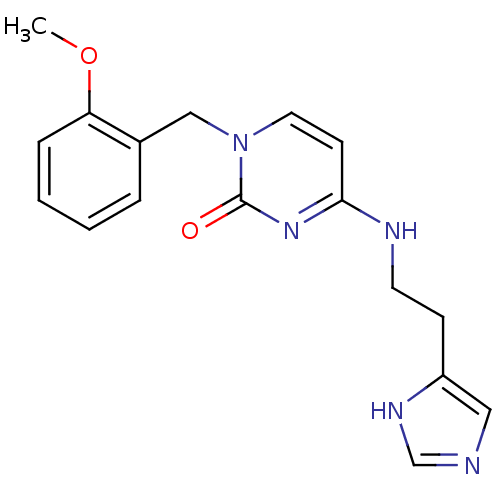
(CHEMBL1916493)Show InChI InChI=1S/C17H19N5O2/c1-24-15-5-3-2-4-13(15)11-22-9-7-16(21-17(22)23)19-8-6-14-10-18-12-20-14/h2-5,7,9-10,12H,6,8,11H2,1H3,(H,18,20)(H,19,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357266
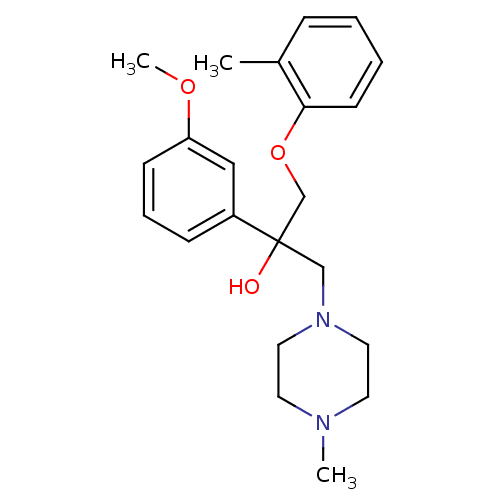
(CHEMBL1916492)Show InChI InChI=1S/C22H30N2O3/c1-18-7-4-5-10-21(18)27-17-22(25,16-24-13-11-23(2)12-14-24)19-8-6-9-20(15-19)26-3/h4-10,15,25H,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357267

(CHEMBL1916493)Show InChI InChI=1S/C17H19N5O2/c1-24-15-5-3-2-4-13(15)11-22-9-7-16(21-17(22)23)19-8-6-14-10-18-12-20-14/h2-5,7,9-10,12H,6,8,11H2,1H3,(H,18,20)(H,19,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356889
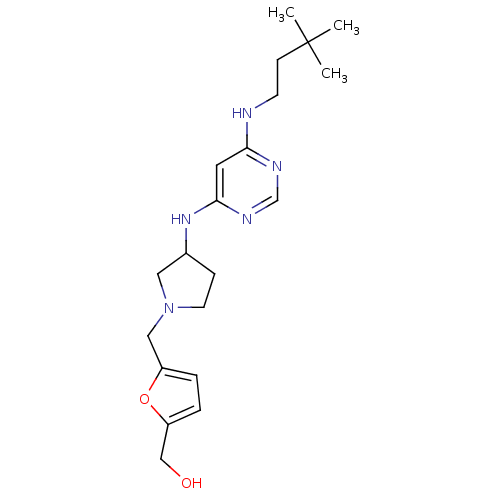
(CHEMBL1915532)Show SMILES CC(C)(C)CCNc1cc(NC2CCN(Cc3ccc(CO)o3)C2)ncn1 Show InChI InChI=1S/C20H31N5O2/c1-20(2,3)7-8-21-18-10-19(23-14-22-18)24-15-6-9-25(11-15)12-16-4-5-17(13-26)27-16/h4-5,10,14-15,26H,6-9,11-13H2,1-3H3,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357266

(CHEMBL1916492)Show InChI InChI=1S/C22H30N2O3/c1-18-7-4-5-10-21(18)27-17-22(25,16-24-13-11-23(2)12-14-24)19-8-6-9-20(15-19)26-3/h4-10,15,25H,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357266

(CHEMBL1916492)Show InChI InChI=1S/C22H30N2O3/c1-18-7-4-5-10-21(18)27-17-22(25,16-24-13-11-23(2)12-14-24)19-8-6-9-20(15-19)26-3/h4-10,15,25H,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H2 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H2 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357268

(CHEMBL1916494)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357270
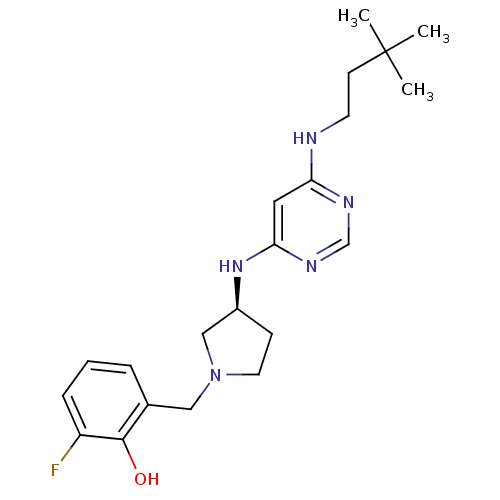
(CHEMBL1916496)Show SMILES CC(C)(C)CCNc1cc(N[C@H]2CCN(Cc3cccc(F)c3O)C2)ncn1 |r| Show InChI InChI=1S/C21H30FN5O/c1-21(2,3)8-9-23-18-11-19(25-14-24-18)26-16-7-10-27(13-16)12-15-5-4-6-17(22)20(15)28/h4-6,11,14,16,28H,7-10,12-13H2,1-3H3,(H2,23,24,25,26)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357269

(CHEMBL1916495)Show SMILES COCc1ccc(CN2CC[C@@H](C2)Nc2cc(NCCC(C)(C)C)ncn2)o1 |r| Show InChI InChI=1S/C21H33N5O2/c1-21(2,3)8-9-22-19-11-20(24-15-23-19)25-16-7-10-26(12-16)13-17-5-6-18(28-17)14-27-4/h5-6,11,15-16H,7-10,12-14H2,1-4H3,(H2,22,23,24,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357265

(CHEMBL1916491)Show SMILES CN1CCN(CC1)c1cc(ncn1)C(=O)NCc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C18H20F3N5O/c1-25-5-7-26(8-6-25)16-10-15(23-12-24-16)17(27)22-11-13-3-2-4-14(9-13)18(19,20)21/h2-4,9-10,12H,5-8,11H2,1H3,(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data