 Found 31 hits of Enzyme Inhibition Constant Data
Found 31 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356004
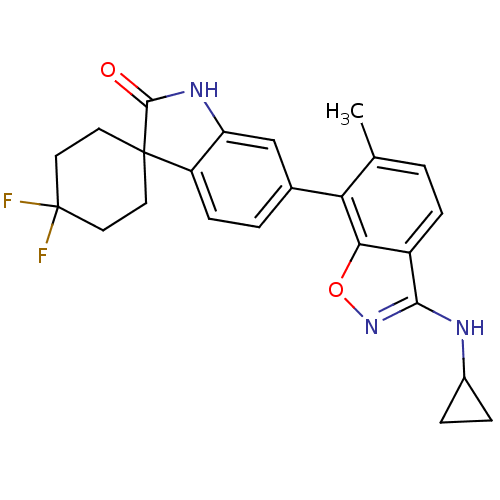
(CHEMBL1911339)Show SMILES Cc1ccc2c(NC3CC3)noc2c1-c1ccc2c(NC(=O)C22CCC(F)(F)CC2)c1 Show InChI InChI=1S/C24H23F2N3O2/c1-13-2-6-16-20(31-29-21(16)27-15-4-5-15)19(13)14-3-7-17-18(12-14)28-22(30)23(17)8-10-24(25,26)11-9-23/h2-3,6-7,12,15H,4-5,8-11H2,1H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356003
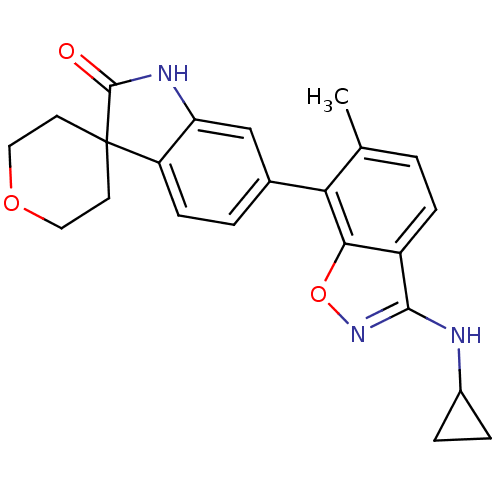
(CHEMBL1911338)Show SMILES Cc1ccc2c(NC3CC3)noc2c1-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C23H23N3O3/c1-13-2-6-16-20(29-26-21(16)24-15-4-5-15)19(13)14-3-7-17-18(12-14)25-22(27)23(17)8-10-28-11-9-23/h2-3,6-7,12,15H,4-5,8-11H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356002
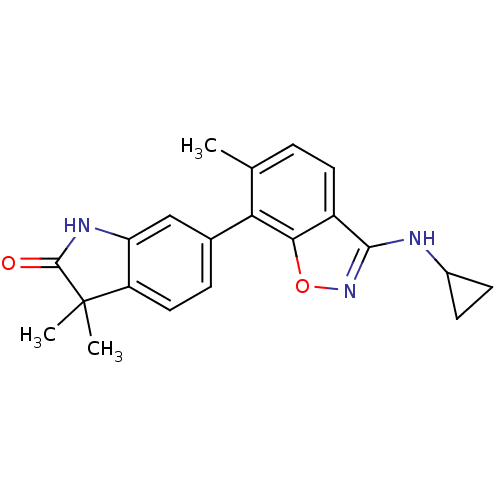
(CHEMBL1911337)Show SMILES Cc1ccc2c(NC3CC3)noc2c1-c1ccc2c(NC(=O)C2(C)C)c1 Show InChI InChI=1S/C21H21N3O2/c1-11-4-8-14-18(26-24-19(14)22-13-6-7-13)17(11)12-5-9-15-16(10-12)23-20(25)21(15,2)3/h4-5,8-10,13H,6-7H2,1-3H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355999
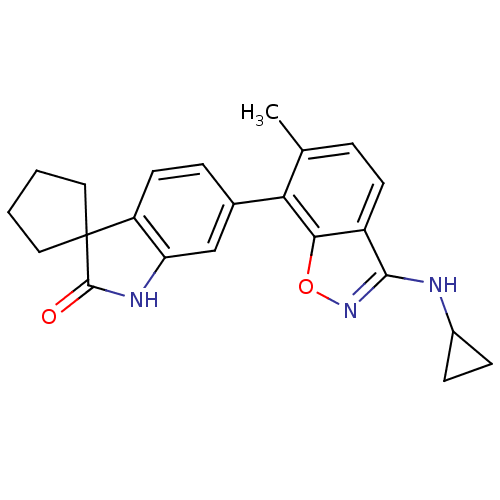
(CHEMBL1911334)Show SMILES Cc1ccc2c(NC3CC3)noc2c1-c1ccc2c(NC(=O)C22CCCC2)c1 Show InChI InChI=1S/C23H23N3O2/c1-13-4-8-16-20(28-26-21(16)24-15-6-7-15)19(13)14-5-9-17-18(12-14)25-22(27)23(17)10-2-3-11-23/h4-5,8-9,12,15H,2-3,6-7,10-11H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356007
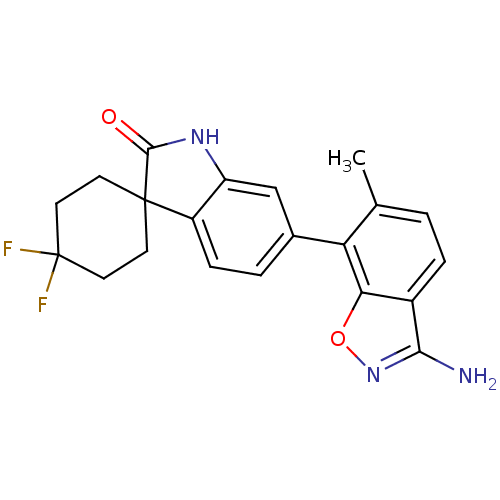
(CHEMBL1911342)Show SMILES Cc1ccc2c(N)noc2c1-c1ccc2c(NC(=O)C22CCC(F)(F)CC2)c1 Show InChI InChI=1S/C21H19F2N3O2/c1-11-2-4-13-17(28-26-18(13)24)16(11)12-3-5-14-15(10-12)25-19(27)20(14)6-8-21(22,23)9-7-20/h2-5,10H,6-9H2,1H3,(H2,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355997
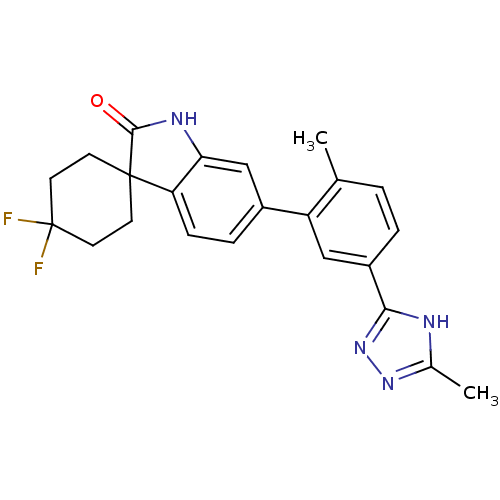
(CHEMBL1911332 | US8772288, 63)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCC(F)(F)CC2)c1 Show InChI InChI=1S/C23H22F2N4O/c1-13-3-4-16(20-26-14(2)28-29-20)11-17(13)15-5-6-18-19(12-15)27-21(30)22(18)7-9-23(24,25)10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,27,30)(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356001
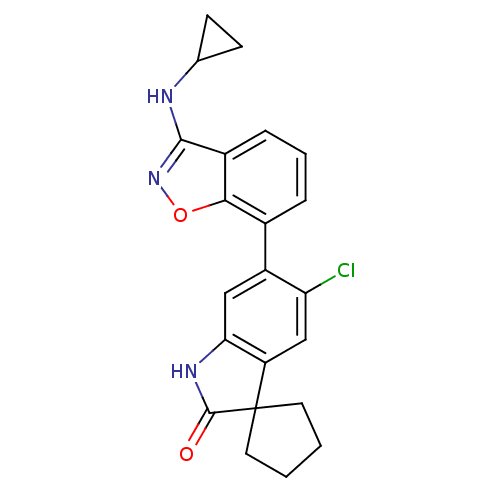
(CHEMBL1911336)Show SMILES Clc1cc2c(NC(=O)C22CCCC2)cc1-c1cccc2c(NC3CC3)noc12 Show InChI InChI=1S/C22H20ClN3O2/c23-17-11-16-18(25-21(27)22(16)8-1-2-9-22)10-15(17)13-4-3-5-14-19(13)28-26-20(14)24-12-6-7-12/h3-5,10-12H,1-2,6-9H2,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355998
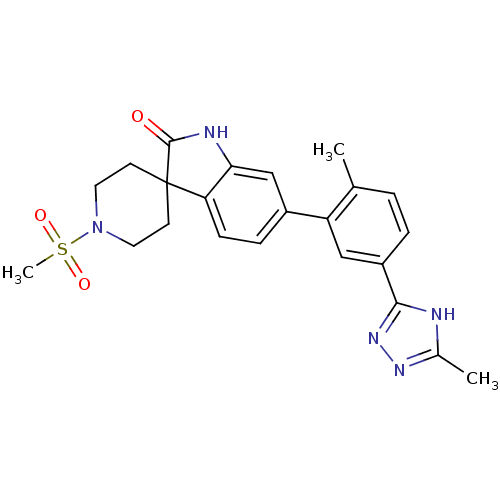
(CHEMBL1911333 | US8772288, 67)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCN(CC2)S(C)(=O)=O)c1 Show InChI InChI=1S/C23H25N5O3S/c1-14-4-5-17(21-24-15(2)26-27-21)12-18(14)16-6-7-19-20(13-16)25-22(29)23(19)8-10-28(11-9-23)32(3,30)31/h4-7,12-13H,8-11H2,1-3H3,(H,25,29)(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356006
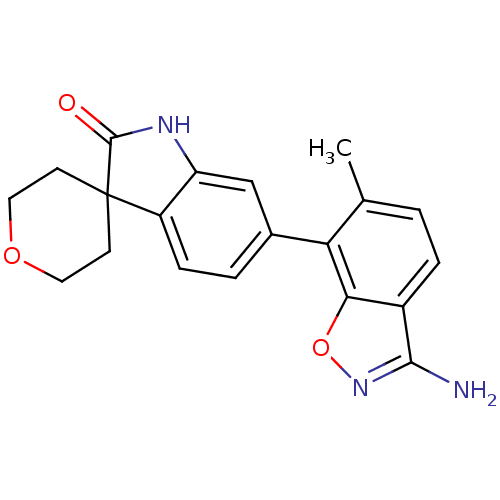
(CHEMBL1911341)Show SMILES Cc1ccc2c(N)noc2c1-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C20H19N3O3/c1-11-2-4-13-17(26-23-18(13)21)16(11)12-3-5-14-15(10-12)22-19(24)20(14)6-8-25-9-7-20/h2-5,10H,6-9H2,1H3,(H2,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355989
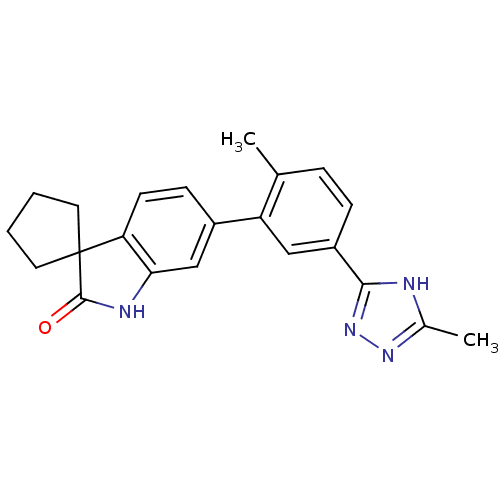
(CHEMBL1911193 | US8772288, 53)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCCC2)c1 Show InChI InChI=1S/C22H22N4O/c1-13-5-6-16(20-23-14(2)25-26-20)11-17(13)15-7-8-18-19(12-15)24-21(27)22(18)9-3-4-10-22/h5-8,11-12H,3-4,9-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355990
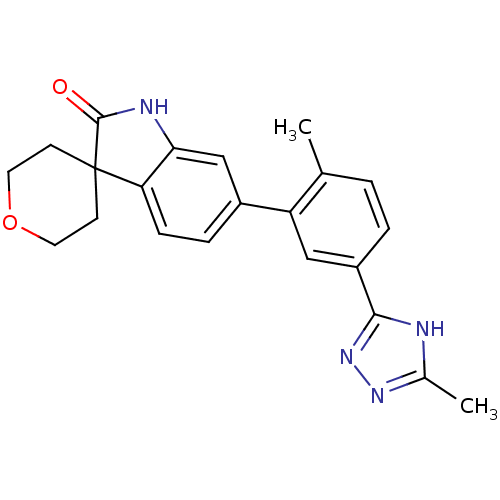
(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355993
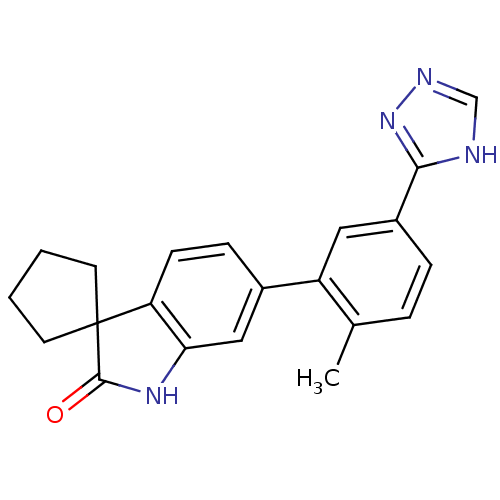
(CHEMBL1911197)Show SMILES Cc1ccc(cc1-c1ccc2c(NC(=O)C22CCCC2)c1)-c1nnc[nH]1 Show InChI InChI=1S/C21H20N4O/c1-13-4-5-15(19-22-12-23-25-19)10-16(13)14-6-7-17-18(11-14)24-20(26)21(17)8-2-3-9-21/h4-7,10-12H,2-3,8-9H2,1H3,(H,24,26)(H,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355995
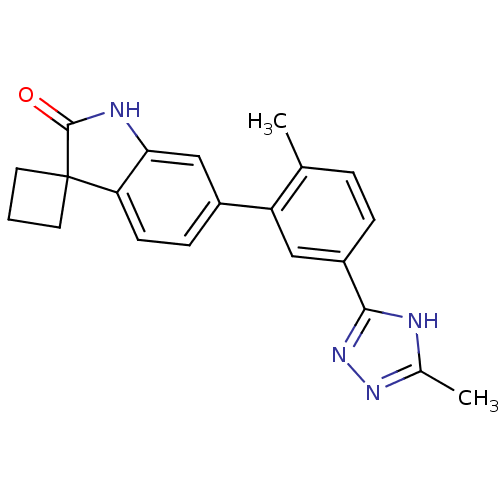
(CHEMBL1909657 | US8772288, 54)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCC2)c1 Show InChI InChI=1S/C21H20N4O/c1-12-4-5-15(19-22-13(2)24-25-19)10-16(12)14-6-7-17-18(11-14)23-20(26)21(17)8-3-9-21/h4-7,10-11H,3,8-9H2,1-2H3,(H,23,26)(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355986

(CHEMBL1911190 | US8772288, 70)Show SMILES Cc1nnc(o1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCCC2)c1 Show InChI InChI=1S/C22H21N3O2/c1-13-5-6-16(20-25-24-14(2)27-20)11-17(13)15-7-8-18-19(12-15)23-21(26)22(18)9-3-4-10-22/h5-8,11-12H,3-4,9-10H2,1-2H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356008
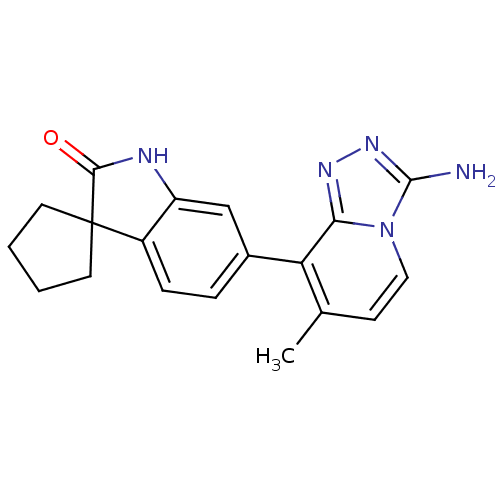
(CHEMBL1911344)Show SMILES Cc1ccn2c(N)nnc2c1-c1ccc2c(NC(=O)C22CCCC2)c1 Show InChI InChI=1S/C19H19N5O/c1-11-6-9-24-16(22-23-18(24)20)15(11)12-4-5-13-14(10-12)21-17(25)19(13)7-2-3-8-19/h4-6,9-10H,2-3,7-8H2,1H3,(H2,20,23)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356000
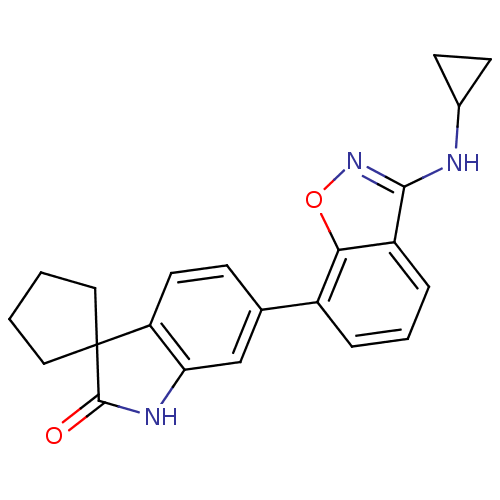
(CHEMBL1911335)Show SMILES O=C1Nc2cc(ccc2C11CCCC1)-c1cccc2c(NC3CC3)noc12 Show InChI InChI=1S/C22H21N3O2/c26-21-22(10-1-2-11-22)17-9-6-13(12-18(17)24-21)15-4-3-5-16-19(15)27-25-20(16)23-14-7-8-14/h3-6,9,12,14H,1-2,7-8,10-11H2,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50356005
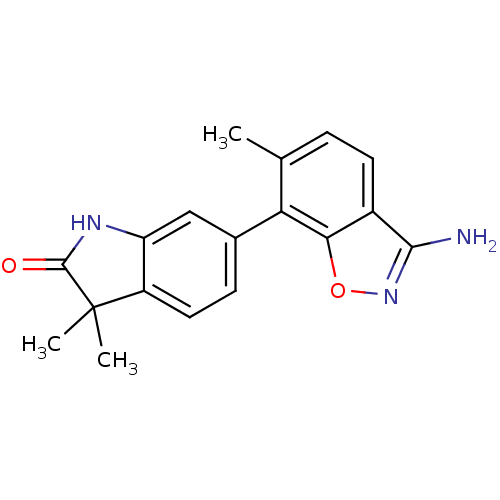
(CHEMBL1911340)Show InChI InChI=1S/C18H17N3O2/c1-9-4-6-11-15(23-21-16(11)19)14(9)10-5-7-12-13(8-10)20-17(22)18(12,2)3/h4-8H,1-3H3,(H2,19,21)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355996

(CHEMBL1911331)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C2(C)C)c1 Show InChI InChI=1S/C20H20N4O/c1-11-5-6-14(18-21-12(2)23-24-18)9-15(11)13-7-8-16-17(10-13)22-19(25)20(16,3)4/h5-10H,1-4H3,(H,22,25)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355994

(CHEMBL1911198)Show SMILES Cc1ccc(cc1-c1ccc2c(NC(=O)C22CCCC2)c1)-c1nnc([nH]1)C1CC1 Show InChI InChI=1S/C24H24N4O/c1-14-4-5-17(22-26-21(27-28-22)15-6-7-15)12-18(14)16-8-9-19-20(13-16)25-23(29)24(19)10-2-3-11-24/h4-5,8-9,12-13,15H,2-3,6-7,10-11H2,1H3,(H,25,29)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355991

(CHEMBL1911195)Show SMILES Cc1c[nH]c(n1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C23H23N3O2/c1-14-3-4-17(21-24-13-15(2)25-21)11-18(14)16-5-6-19-20(12-16)26-22(27)23(19)7-9-28-10-8-23/h3-6,11-13H,7-10H2,1-2H3,(H,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355987

(CHEMBL1911191)Show SMILES Cc1nc(no1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCCC2)c1 Show InChI InChI=1S/C22H21N3O2/c1-13-5-6-16(20-23-14(2)27-25-20)11-17(13)15-7-8-18-19(12-15)24-21(26)22(18)9-3-4-10-22/h5-8,11-12H,3-4,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 583 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355988

(CHEMBL1911192)Show SMILES Cc1noc(n1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCCC2)c1 Show InChI InChI=1S/C22H21N3O2/c1-13-5-6-16(20-23-14(2)25-27-20)11-17(13)15-7-8-18-19(12-15)24-21(26)22(18)9-3-4-10-22/h5-8,11-12H,3-4,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50355992

(CHEMBL1911196)Show SMILES Cc1nc(c[nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C23H23N3O2/c1-14-3-4-17(21-13-24-15(2)25-21)11-18(14)16-5-6-19-20(12-16)26-22(27)23(19)7-9-28-10-8-23/h3-6,11-13H,7-10H2,1-2H3,(H,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha using biotinylated ATF2 as substrate preincubated for 1 hr measured after 1 hr by FRET assay |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cRaf |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human TrkA |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT1B |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50355990

(CHEMBL1911194 | US8772288, 59)Show SMILES Cc1nnc([nH]1)-c1ccc(C)c(c1)-c1ccc2c(NC(=O)C22CCOCC2)c1 Show InChI InChI=1S/C22H22N4O2/c1-13-3-4-16(20-23-14(2)25-26-20)11-17(13)15-5-6-18-19(12-15)24-21(27)22(18)7-9-28-10-8-22/h3-6,11-12H,7-10H2,1-2H3,(H,24,27)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 21: 6253-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.006
BindingDB Entry DOI: 10.7270/Q25B02W3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data