

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM55094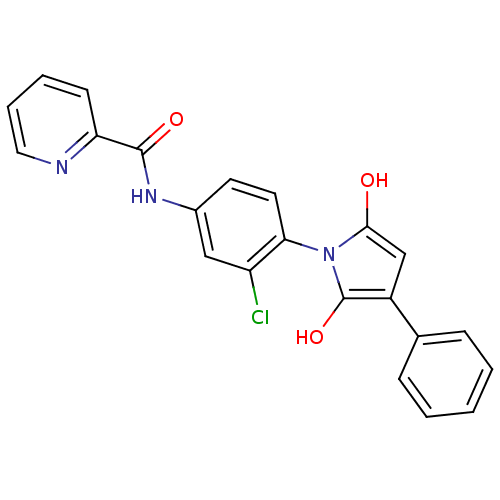 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using 4-hydroxydiclofenac as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM55094 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using acetaminophen as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM55094 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50358179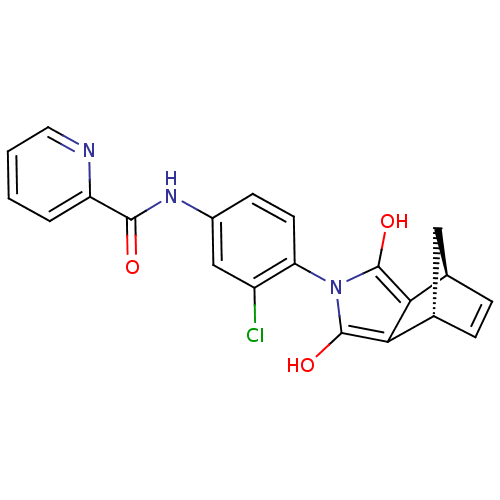 (CHEMBL1921961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using 1-hydroxymidazolam as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50358174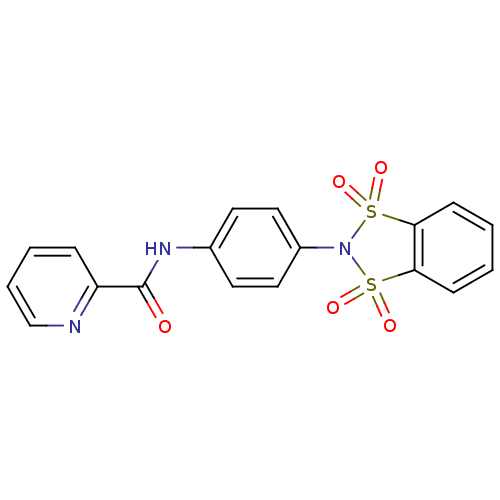 (CHEMBL1921855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using 1-hydroxymidazolam as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM55094 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextrophan tartarate as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50358174 (CHEMBL1921855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using 4-hydroxydiclofenac as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50358179 (CHEMBL1921961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using acetaminophen as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50358174 (CHEMBL1921855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using acetaminophen as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM55093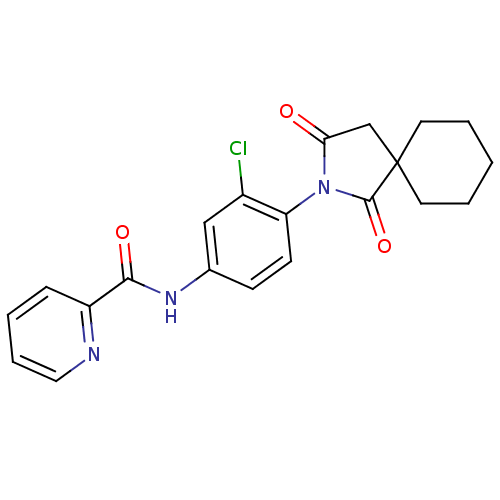 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using 1-hydroxymidazolam as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM55093 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextrophan tartarate as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50358179 (CHEMBL1921961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextrophan tartarate as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM55093 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using 4-hydroxydiclofenac as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50358179 (CHEMBL1921961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM55094 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using 1-hydroxymidazolam as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50358179 (CHEMBL1921961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using 4-hydroxydiclofenac as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM55093 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using acetaminophen as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50293721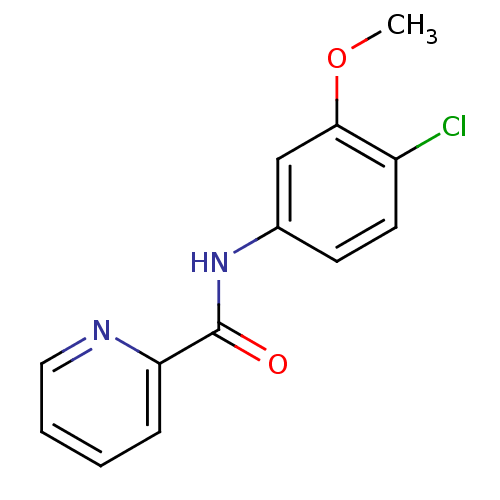 (CHEMBL562551 | N-(4-chloro-3-methoxyphenyl)picolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using 1-hydroxymidazolam as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50358174 (CHEMBL1921855) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextrophan tartarate as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293721 (CHEMBL562551 | N-(4-chloro-3-methoxyphenyl)picolin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextrophan tartarate as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50293721 (CHEMBL562551 | N-(4-chloro-3-methoxyphenyl)picolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using 4-hydroxydiclofenac as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM55093 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM55094 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 491 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM55095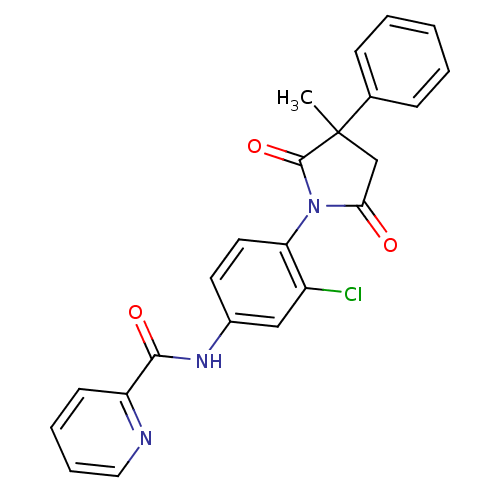 (N-[3-chloranyl-4-[3-methyl-2,5-bis(oxidanylidene)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 536 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM55086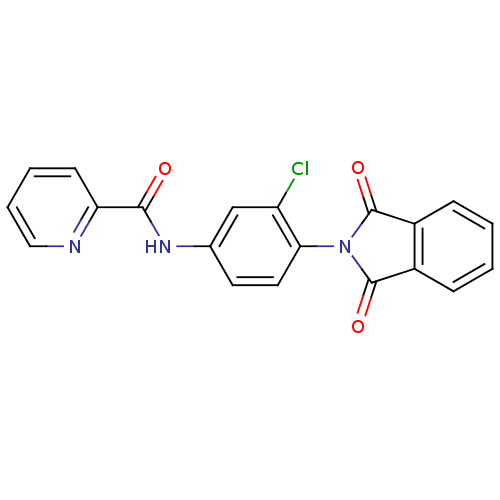 (N-(3-chloro-4-phthalimido-phenyl)picolinamide | N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 66.8 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM55087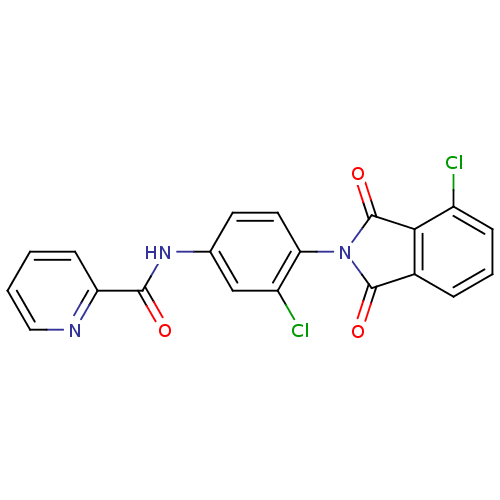 (N-[3-chloranyl-4-[4-chloranyl-1,3-bis(oxidanyliden...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 34.9 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358176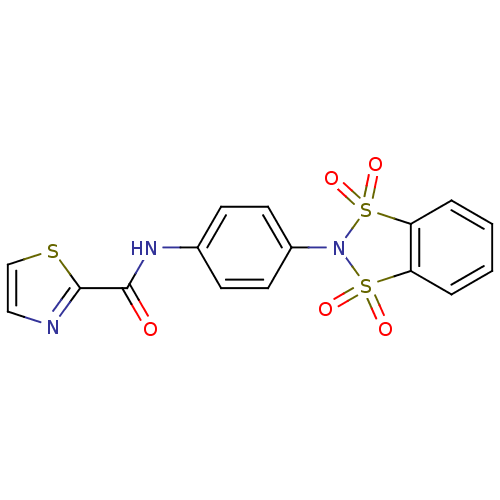 (CHEMBL1921951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 353 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55086 (N-(3-chloro-4-phthalimido-phenyl)picolinamide | N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 59.4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358174 (CHEMBL1921855) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358179 (CHEMBL1921961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 376 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358182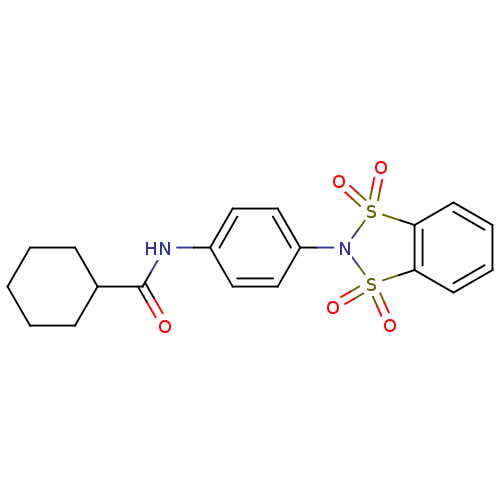 (CHEMBL1921854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 765 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358184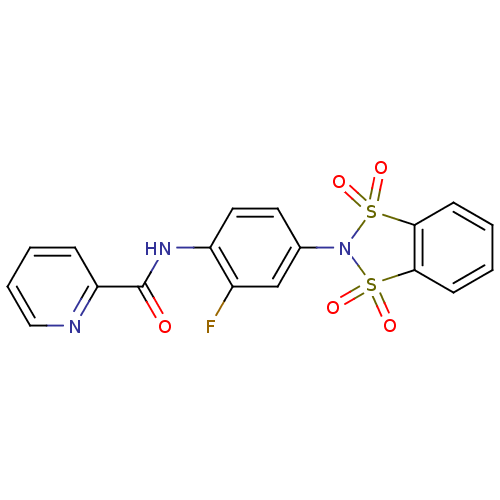 (CHEMBL1921954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 7 (Rattus norvegicus (Rat)) | BDBM50358179 (CHEMBL1921961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu7 receptor at 10 uM by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358176 (CHEMBL1921951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 201 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358185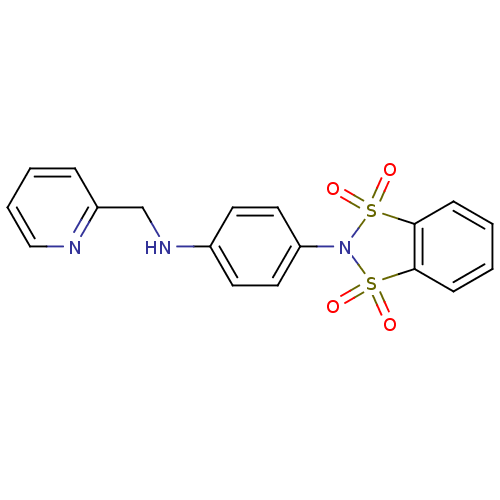 (CHEMBL1921955) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358178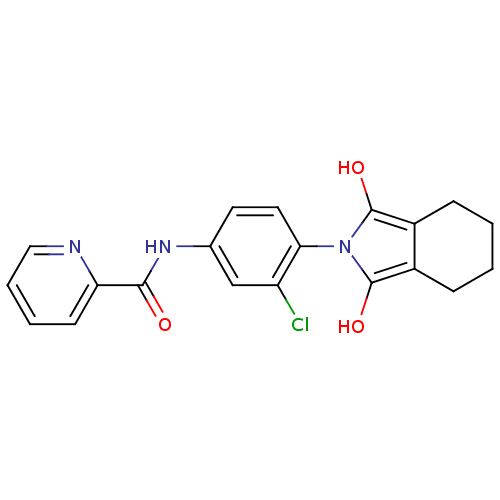 (CHEMBL1921959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358187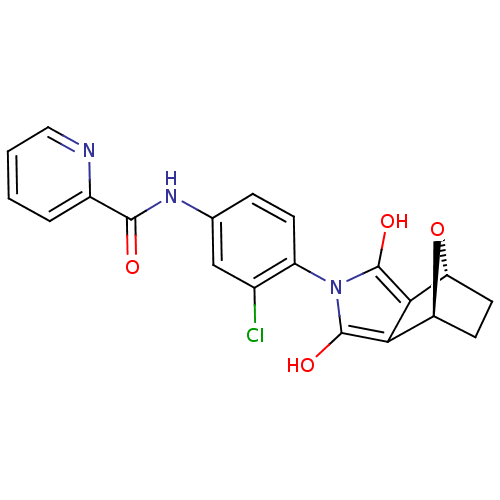 (CHEMBL1921960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50358179 (CHEMBL1921961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu5 receptor expressed in human HEK293 at 10 uM by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55095 (N-[3-chloranyl-4-[3-methyl-2,5-bis(oxidanylidene)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 771 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358173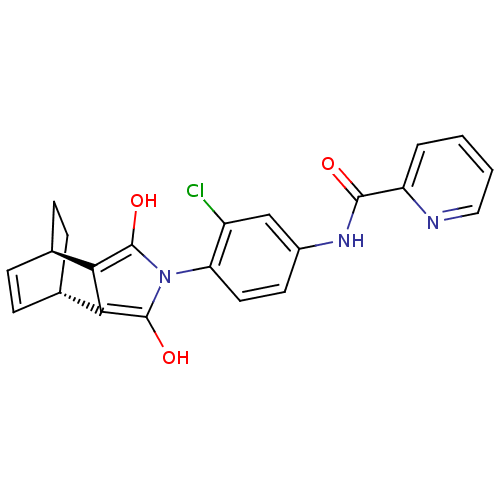 (CHEMBL1921962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 287 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM55093 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 397 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM55091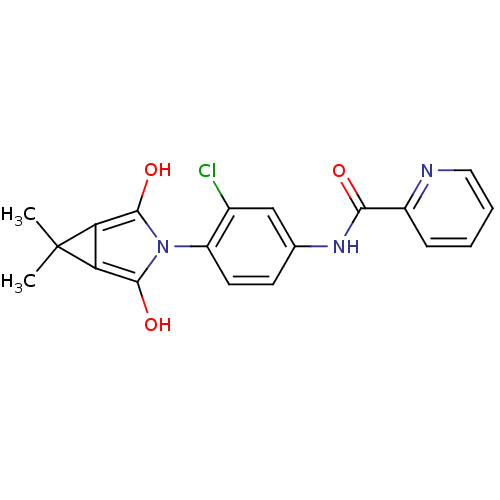 (N-[3-chloranyl-4-[(1S,5R)-6,6-dimethyl-2,4-bis(oxi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 686 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358173 (CHEMBL1921962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 246 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358177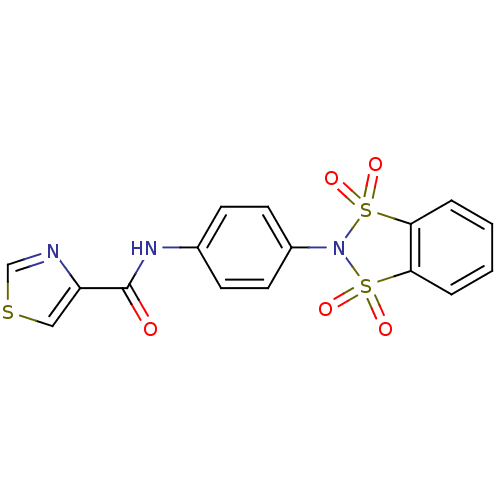 (CHEMBL1921952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358175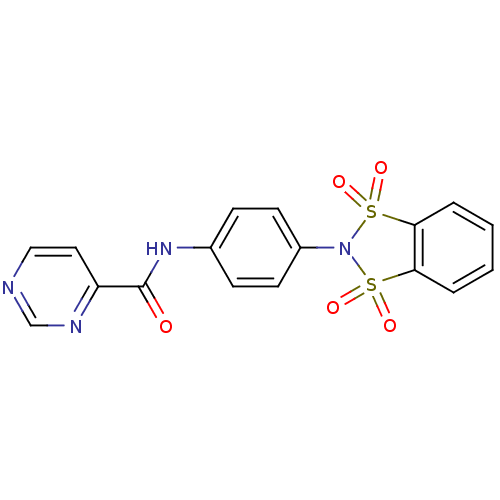 (CHEMBL1921950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 242 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55092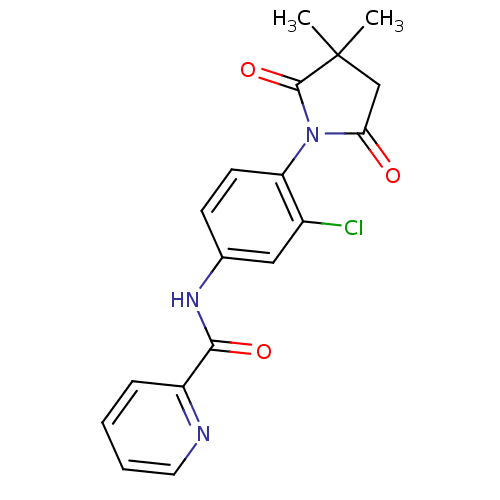 (N-[3-chloranyl-4-[3,3-dimethyl-2,5-bis(oxidanylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358179 (CHEMBL1921961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 291 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55087 (N-[3-chloranyl-4-[4-chloranyl-1,3-bis(oxidanyliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 41.9 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55091 (N-[3-chloranyl-4-[(1S,5R)-6,6-dimethyl-2,4-bis(oxi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 435 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55085 (N-(3-chloro-4-succinimido-phenyl)picolinamide | N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358186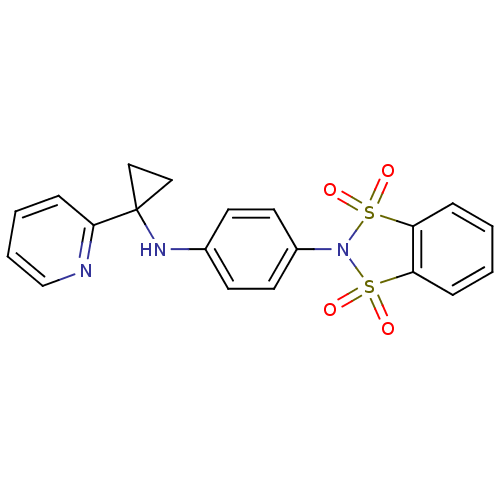 (CHEMBL1921956) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358175 (CHEMBL1921950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 179 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358180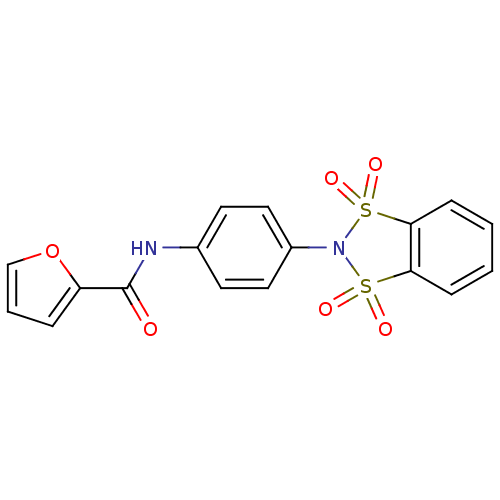 (CHEMBL1921852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 226 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55093 (N-[3-chloro-4-(1,3-diketo-2-azaspiro[4.5]decan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358177 (CHEMBL1921952) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 104 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358183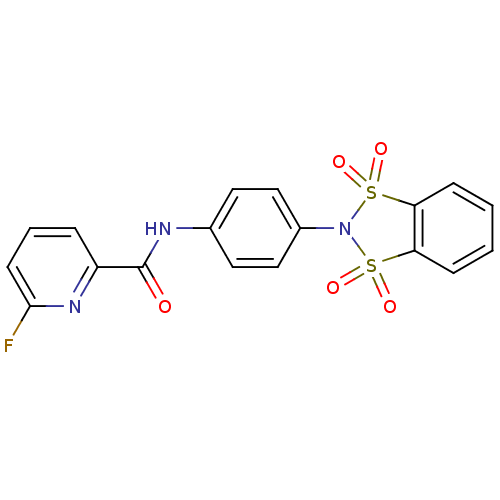 (CHEMBL1921948) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358174 (CHEMBL1921855) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 136 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50358181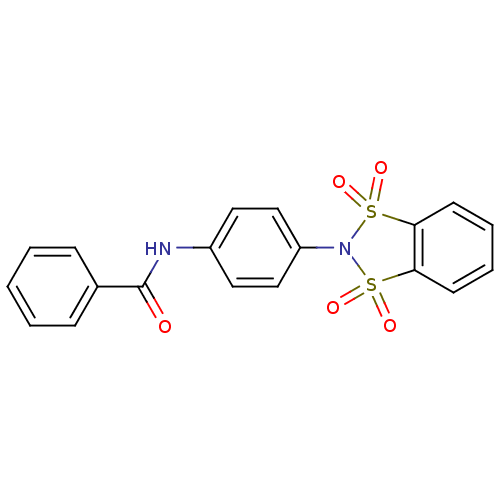 (CHEMBL1921853) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 402 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 6 (Homo sapiens (Human)) | BDBM50358179 (CHEMBL1921961) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu6 receptor at 10 uM by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM55094 (N-[3-chloro-4-(2,5-diketo-3-phenyl-pyrrolidino)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 674 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human mGlu4 receptor expressed in CHO cells expressing Gqi5 by calcium mobilization assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50358178 (CHEMBL1921959) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 645 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at rat mGlu4 receptor by thallium flux assay | J Med Chem 54: 7639-47 (2011) Article DOI: 10.1021/jm200956q BindingDB Entry DOI: 10.7270/Q25Q4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||