

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364063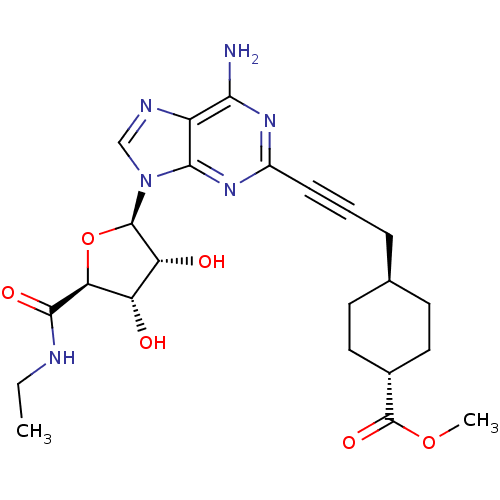 (CHEMBL1950649) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364061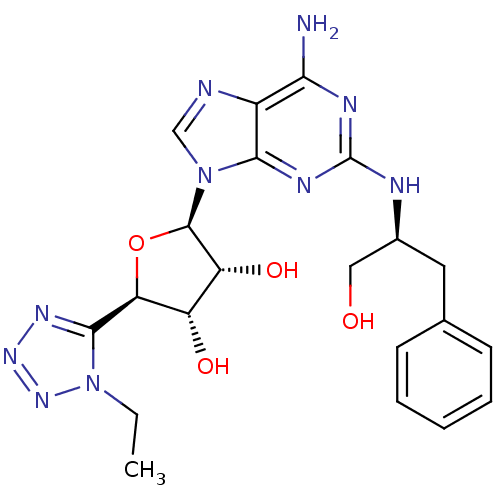 (CHEMBL1950652) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human A2A adenosine receptor expressed in CHO cells | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364044 (CHEMBL260203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316212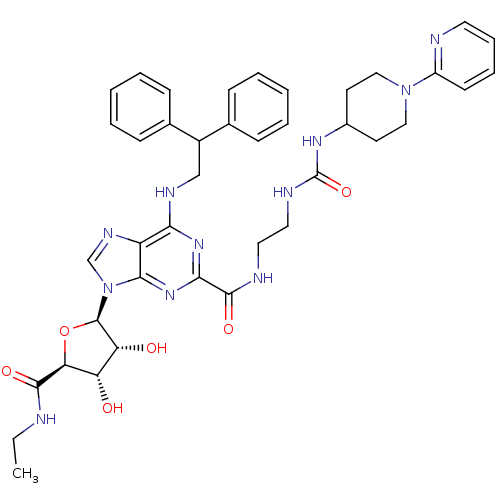 (6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50119168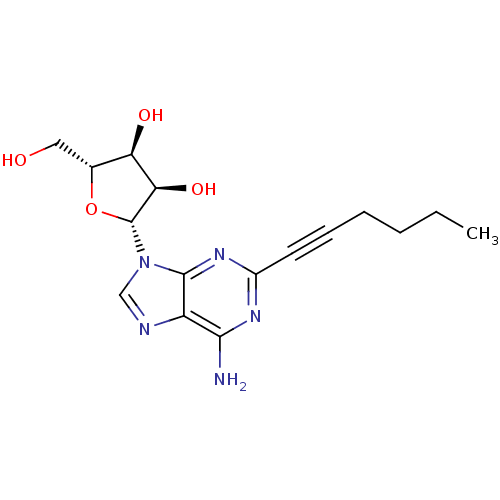 ((2R,3R,4S,5R)-2-(6-Amino-2-hex-1-ynyl-purin-9-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50364060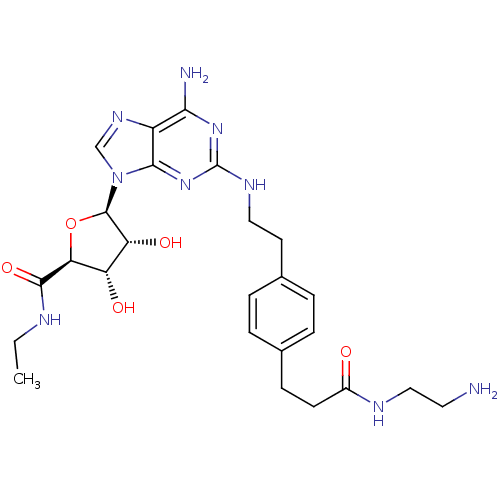 (CHEMBL1950647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]NECA from A2A adenosine receptor expressed in rat striatal membranes after 30 mins by liquid scintillation counting | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50009263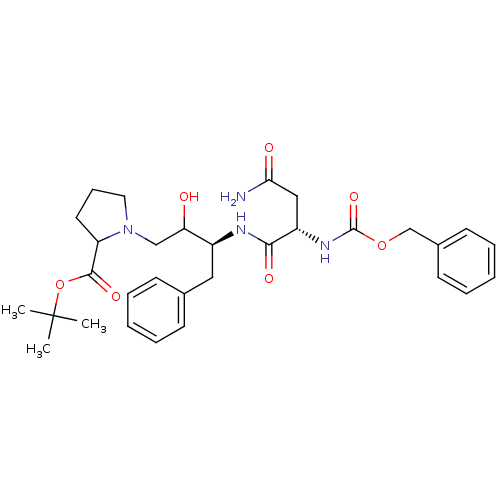 ((2S,3S,4R,5R)-5-(6-amino-2-(hex-1-ynyl)-9H-purin-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339076 ((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233810 (9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from A2A adenosine receptor expressed in rat striatal membranes after 30 mins by liquid scintillation counting | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364056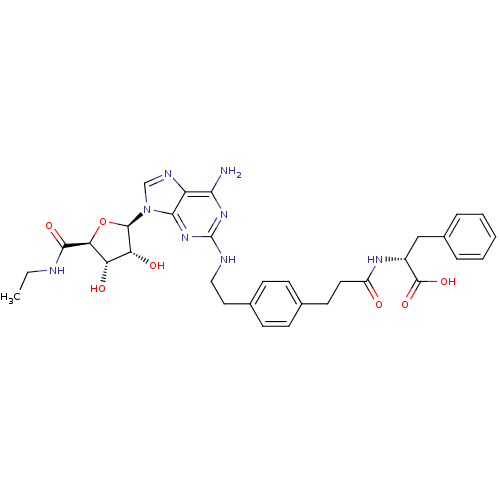 (CHEMBL1950662) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364046 (CHEMBL1950666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50163015 ((2R,3R,4S,5R)-2-[6-(2,2-Diphenyl-ethylamino)-purin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364054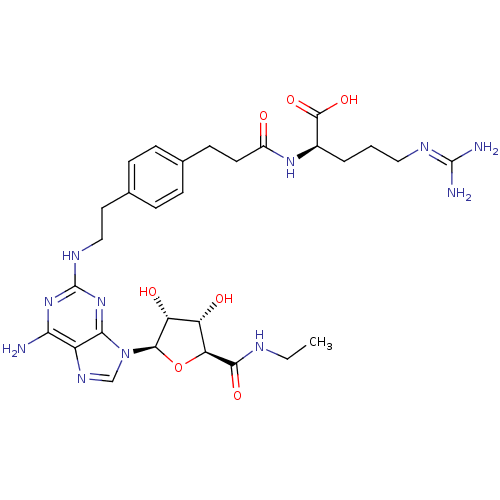 (CHEMBL1950660) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364064 (CHEMBL1950650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364043 (CHEMBL1950553) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 58.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364055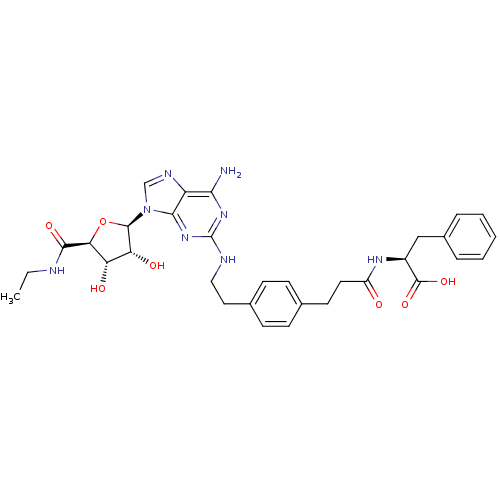 (CHEMBL1950661) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364057 (CHEMBL1950663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364048 (CHEMBL1950654) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364049 (CHEMBL1950655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 87.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364053 (CHEMBL1950659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364052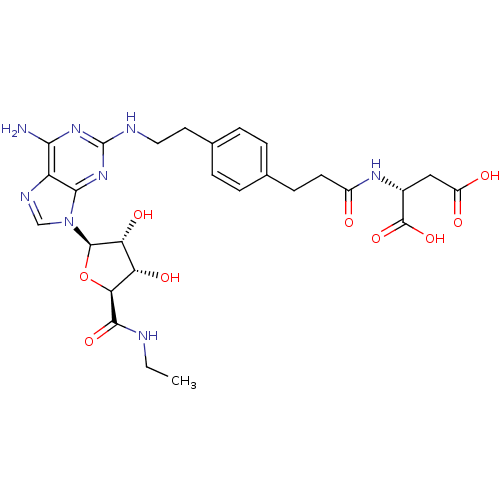 (CHEMBL1950658) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364059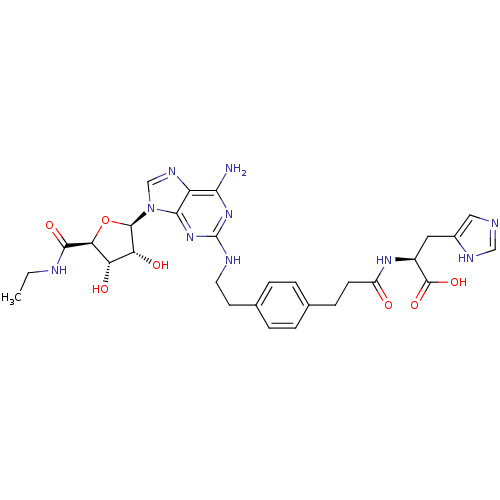 (CHEMBL1950665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364058 (CHEMBL1950664) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364047 (CHEMBL1950653) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364050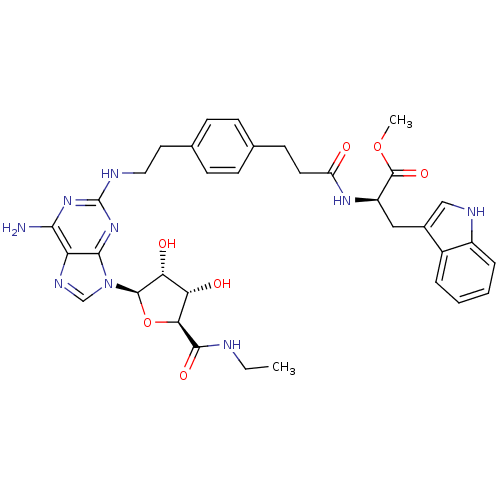 (CHEMBL1950656) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364056 (CHEMBL1950662) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364049 (CHEMBL1950655) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364047 (CHEMBL1950653) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364048 (CHEMBL1950654) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364062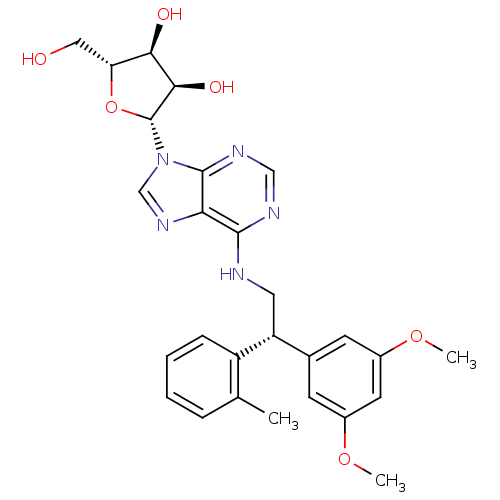 (CHEMBL1950555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364051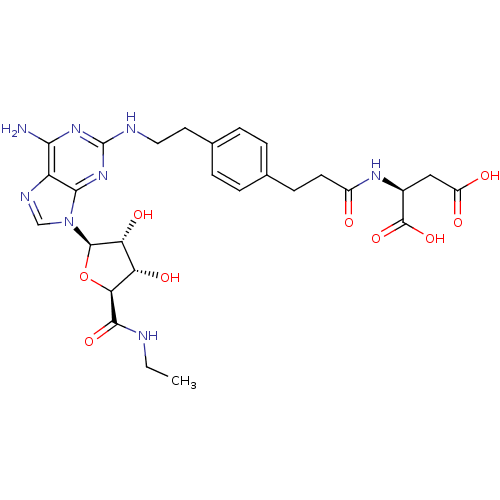 (CHEMBL1950657) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamido-adenosine from human A2A adenosine receptor expressed in HEK293 cell... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364057 (CHEMBL1950663) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364054 (CHEMBL1950660) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364050 (CHEMBL1950656) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364055 (CHEMBL1950661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364045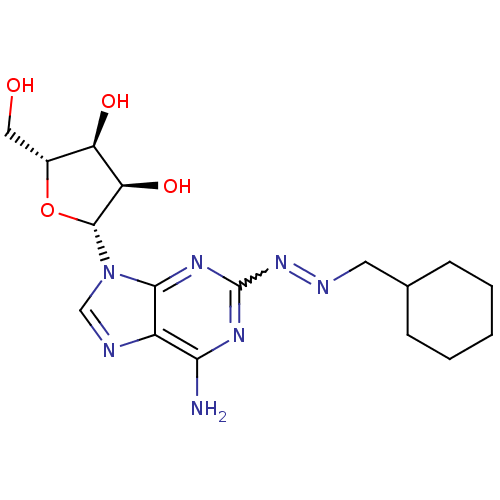 (CHEMBL1950554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50119132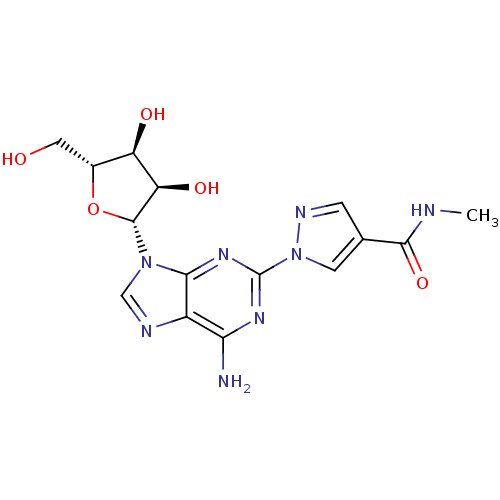 (1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364046 (CHEMBL1950666) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364046 (CHEMBL1950666) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50300876 ((2R,3R,4S,5R)-2-(6-amino-2-(2-cyclohexylethylthio)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to A2A adenosine receptor | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364058 (CHEMBL1950664) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364056 (CHEMBL1950662) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364053 (CHEMBL1950659) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364055 (CHEMBL1950661) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364052 (CHEMBL1950658) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364059 (CHEMBL1950665) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364054 (CHEMBL1950660) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364059 (CHEMBL1950665) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364057 (CHEMBL1950663) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364047 (CHEMBL1950653) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364053 (CHEMBL1950659) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364052 (CHEMBL1950658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364048 (CHEMBL1950654) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50364051 (CHEMBL1950657) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3 adenosine receptor expressed in CHO cell membranes after ... | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364050 (CHEMBL1950656) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364049 (CHEMBL1950655) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364051 (CHEMBL1950657) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50364058 (CHEMBL1950664) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-phenylisopropyladenosine from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins | J Med Chem 55: 538-52 (2012) Article DOI: 10.1021/jm201461q BindingDB Entry DOI: 10.7270/Q22Z160R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||