 Found 122 hits of Enzyme Inhibition Constant Data
Found 122 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364171
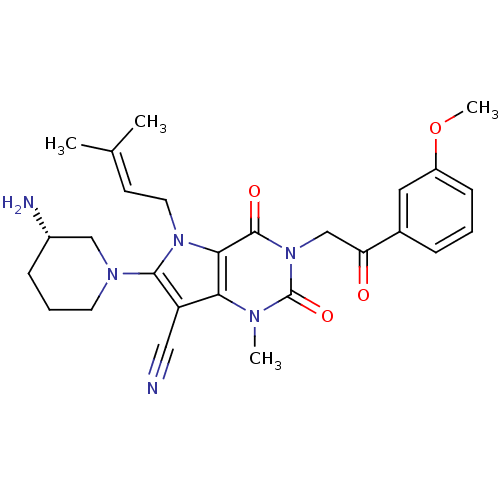
(CHEMBL1951598)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C27H32N6O4/c1-17(2)10-12-32-24-23(21(14-28)25(32)31-11-6-8-19(29)15-31)30(3)27(36)33(26(24)35)16-22(34)18-7-5-9-20(13-18)37-4/h5,7,9-10,13,19H,6,8,11-12,15-16,29H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364156

(CHEMBL1951432)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-7-20(29)15-32)31-17-33(27(25)35)16-23-21-8-4-3-6-19(21)9-11-30-23/h3-4,6,8-11,17,20H,5,7,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364173
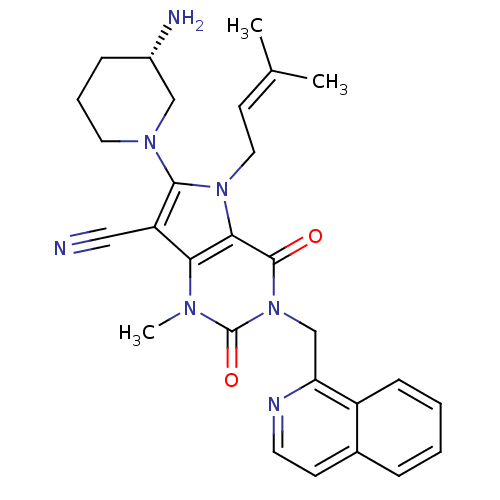
(CHEMBL1951599)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C28H31N7O2/c1-18(2)11-14-34-25-24(22(15-29)26(34)33-13-6-8-20(30)16-33)32(3)28(37)35(27(25)36)17-23-21-9-5-4-7-19(21)10-12-31-23/h4-5,7,9-12,20H,6,8,13-14,16-17,30H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364187

(CHEMBL1951416)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(22(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-23-21(15-29)20-9-6-5-8-19(20)17-32-23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364158

(CHEMBL1951430)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364184
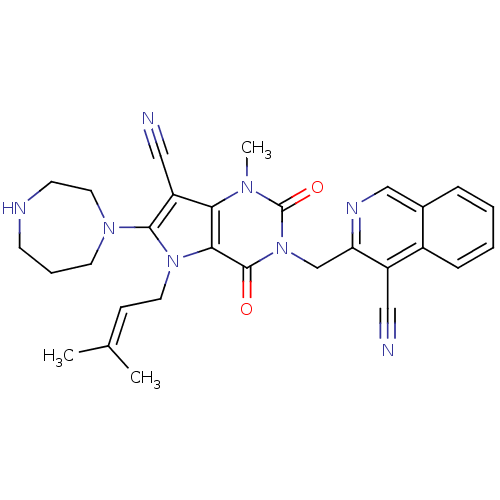
(CHEMBL1951611)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(23(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-24-22(15-30)21-8-5-4-7-20(21)17-33-24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364147

(CHEMBL1951595)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C29H30N6O4/c1-32-25-23(15-30)27(33-13-7-11-21(31)17-33)34(16-19-8-4-3-5-9-19)26(25)28(37)35(29(32)38)18-24(36)20-10-6-12-22(14-20)39-2/h3-6,8-10,12,14,21H,7,11,13,16-18,31H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364179

(CHEMBL1951607)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C27H32N6O4/c1-18(2)9-13-32-24-23(21(16-28)25(32)31-12-6-10-29-11-14-31)30(3)27(36)33(26(24)35)17-22(34)19-7-5-8-20(15-19)37-4/h5,7-9,15,29H,6,10-14,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364183
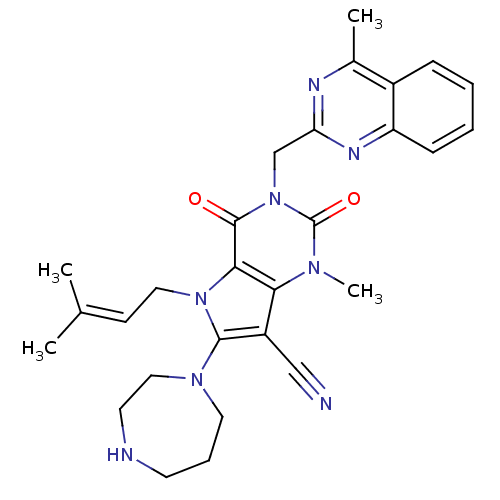
(CHEMBL1951609)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C28H32N8O2/c1-18(2)10-14-35-25-24(21(16-29)26(35)34-13-7-11-30-12-15-34)33(4)28(38)36(27(25)37)17-23-31-19(3)20-8-5-6-9-22(20)32-23/h5-6,8-10,30H,7,11-15,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364167

(CHEMBL1951600)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C28H32N8O2/c1-17(2)11-13-35-25-24(21(14-29)26(35)34-12-7-8-19(30)15-34)33(4)28(38)36(27(25)37)16-23-31-18(3)20-9-5-6-10-22(20)32-23/h5-6,9-11,19H,7-8,12-13,15-16,30H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364177
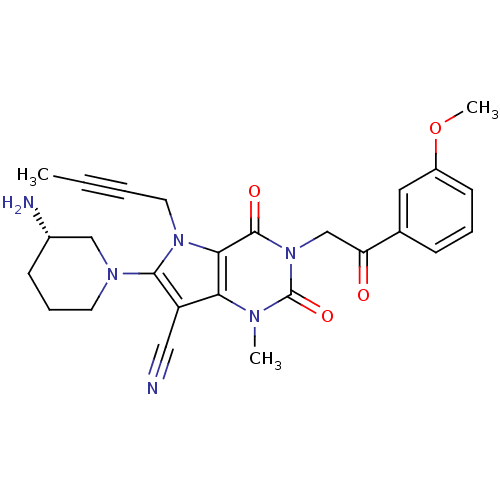
(CHEMBL1951603)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C26H28N6O4/c1-4-5-12-31-23-22(20(14-27)24(31)30-11-7-9-18(28)15-30)29(2)26(35)32(25(23)34)16-21(33)17-8-6-10-19(13-17)36-3/h6,8,10,13,18H,7,9,11-12,15-16,28H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364186
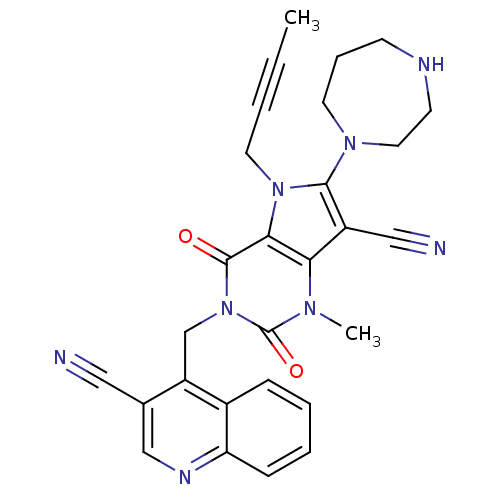
(CHEMBL1951614)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(21(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-22-19(15-29)17-32-23-9-6-5-8-20(22)23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364181
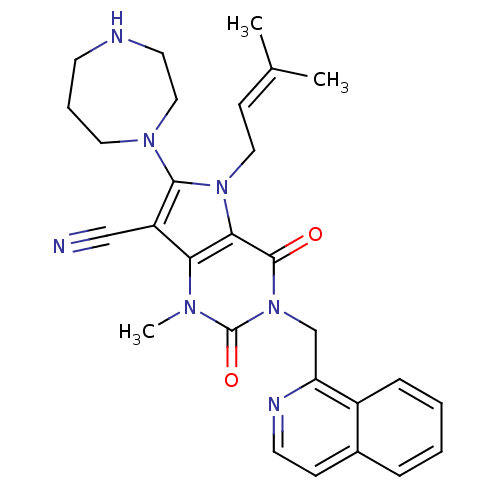
(CHEMBL1951608)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C28H31N7O2/c1-19(2)10-15-34-25-24(22(17-29)26(34)33-14-6-11-30-13-16-33)32(3)28(37)35(27(25)36)18-23-21-8-5-4-7-20(21)9-12-31-23/h4-5,7-10,12,30H,6,11,13-16,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364155

(CHEMBL1951433)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O/c1-17(2)10-12-35-25-24(21(13-28)26(35)33-11-6-7-19(29)14-33)30-16-34(27(25)36)15-23-31-18(3)20-8-4-5-9-22(20)32-23/h4-5,8-10,16,19H,6-7,11-12,14-15,29H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364159
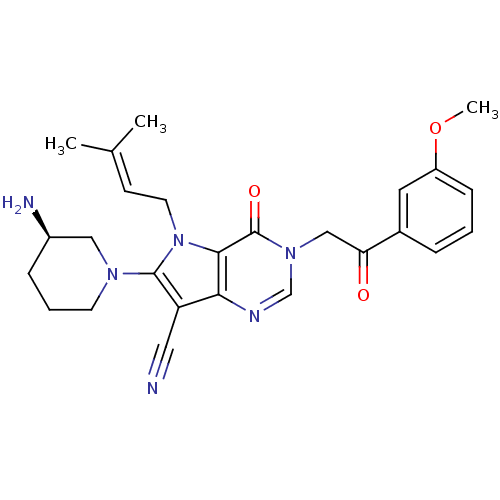
(CHEMBL1951429)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364157

(CHEMBL1951431)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-6-20(29)16-32)31-17-33(27(25)35)15-19-9-11-30-23-8-4-3-7-21(19)23/h3-4,7-11,17,20H,5-6,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364170

(CHEMBL1951596)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(Cc2nccc3ccccc23)c1=O |r| Show InChI InChI=1S/C30H29N7O2/c1-34-26-24(16-31)28(35-15-7-11-22(32)18-35)36(17-20-8-3-2-4-9-20)27(26)29(38)37(30(34)39)19-25-23-12-6-5-10-21(23)13-14-33-25/h2-6,8-10,12-14,22H,7,11,15,17-19,32H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364165

(CHEMBL1951423)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C28H28N6O3/c1-37-22-11-5-9-20(13-22)24(35)17-33-18-31-25-23(14-29)27(32-12-6-10-21(30)16-32)34(26(25)28(33)36)15-19-7-3-2-4-8-19/h2-5,7-9,11,13,18,21H,6,10,12,15-17,30H2,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364176

(CHEMBL1951604)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H27N7O2/c1-3-4-14-33-24-23(21(15-28)25(33)32-13-7-9-19(29)16-32)31(2)27(36)34(26(24)35)17-22-20-10-6-5-8-18(20)11-12-30-22/h5-6,8,10-12,19H,7,9,13-14,16-17,29H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364169

(CHEMBL1951612)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H27N7O2/c1-3-4-15-33-24-23(21(17-28)25(33)32-14-7-11-29-13-16-32)31(2)27(36)34(26(24)35)18-22-20-9-6-5-8-19(20)10-12-30-22/h5-6,8-10,12,29H,7,11,13-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364160

(CHEMBL1951428)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-8-19(28)15-31)30-17-32(26(24)34)16-22-20-9-5-4-7-18(20)10-11-29-22/h4-5,7,9-11,17,19H,6,8,12-13,15-16,28H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364185

(CHEMBL1949693)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(22(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-23-20(15-30)17-33-24-8-5-4-7-21(23)24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364150

(CHEMBL1951438)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C26H25N7O/c1-2-3-14-33-24-23(21(16-27)25(33)31-13-6-10-28-12-15-31)30-18-32(26(24)34)17-22-20-8-5-4-7-19(20)9-11-29-22/h4-5,7-9,11,18,28H,6,10,12-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364152

(CHEMBL1951437)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H29N7O/c1-19(2)9-14-34-25-24(22(16-28)26(34)32-13-5-10-29-12-15-32)31-18-33(27(25)35)17-23-21-7-4-3-6-20(21)8-11-30-23/h3-4,6-9,11,18,29H,5,10,12-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364168
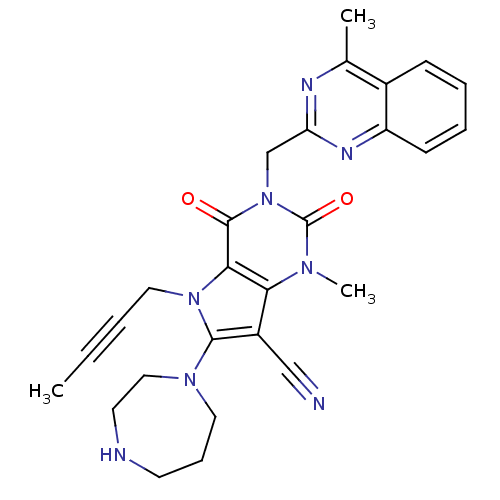
(CHEMBL1951613)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C27H28N8O2/c1-4-5-14-34-24-23(20(16-28)25(34)33-13-8-11-29-12-15-33)32(3)27(37)35(26(24)36)17-22-30-18(2)19-9-6-7-10-21(19)31-22/h6-7,9-10,29H,8,11-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364154
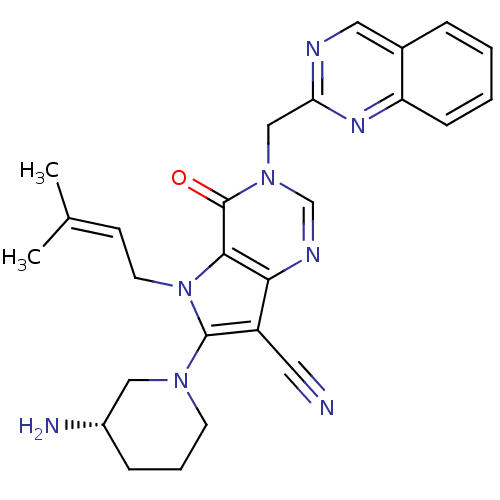
(CHEMBL1951434)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ncc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C26H28N8O/c1-17(2)9-11-34-24-23(20(12-27)25(34)32-10-5-7-19(28)14-32)30-16-33(26(24)35)15-22-29-13-18-6-3-4-8-21(18)31-22/h3-4,6,8-9,13,16,19H,5,7,10-11,14-15,28H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364153
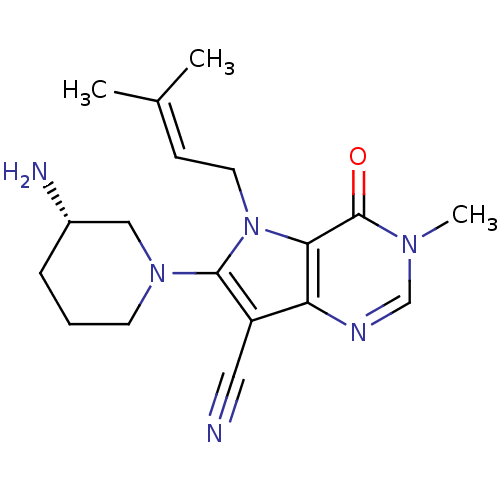
(CHEMBL1951435)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6])c(=O)c12 |r| Show InChI InChI=1S/C18H24N6O/c1-12(2)6-8-24-16-15(21-11-22(3)18(16)25)14(9-19)17(24)23-7-4-5-13(20)10-23/h6,11,13H,4-5,7-8,10,20H2,1-3H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364174

(CHEMBL1951601)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cnc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O2/c1-17(2)10-12-34-24-23(20(13-28)25(34)33-11-6-7-18(29)15-33)32(3)27(37)35(26(24)36)16-19-14-30-21-8-4-5-9-22(21)31-19/h4-5,8-10,14,18H,6-7,11-12,15-16,29H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364148
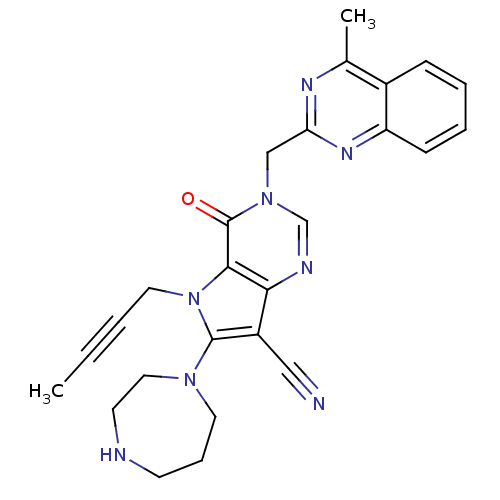
(CHEMBL1951439)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H26N8O/c1-3-4-13-34-24-23(20(15-27)25(34)32-12-7-10-28-11-14-32)29-17-33(26(24)35)16-22-30-18(2)19-8-5-6-9-21(19)31-22/h5-6,8-9,17,28H,7,10-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364172

(CHEMBL1951597)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H24N6O2/c1-24-17-16(11-22)19(26-10-6-9-15(23)13-26)27(12-14-7-4-3-5-8-14)18(17)20(28)25(2)21(24)29/h3-5,7-8,15H,6,9-10,12-13,23H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364182

(CHEMBL1951610)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cn4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H30N8O2/c1-18(2)8-13-33-23-22(20(15-27)24(33)31-12-6-9-28-10-14-31)30(3)26(36)34(25(23)35)17-19-16-32-11-5-4-7-21(32)29-19/h4-5,7-8,11,16,28H,6,9-10,12-14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364161
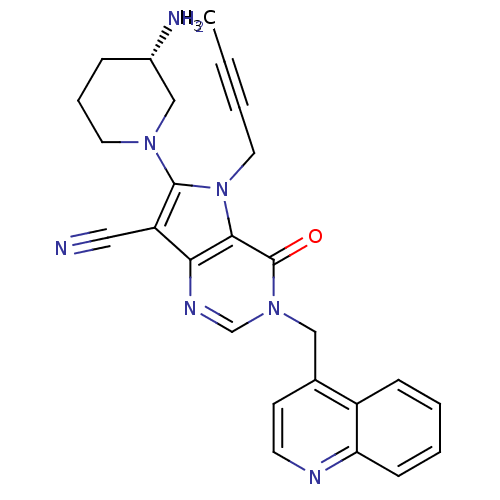
(CHEMBL1951427)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-7-19(28)16-31)30-17-32(26(24)34)15-18-10-11-29-22-9-5-4-8-20(18)22/h4-5,8-11,17,19H,6-7,12-13,15-16,28H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364145

(CHEMBL1951420)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C25H28N6O3/c1-17(2)7-10-31-23-22(20(14-26)24(31)29-11-8-27-9-12-29)28-16-30(25(23)33)15-21(32)18-5-4-6-19(13-18)34-3/h4-7,13,16,27H,8-12,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364162
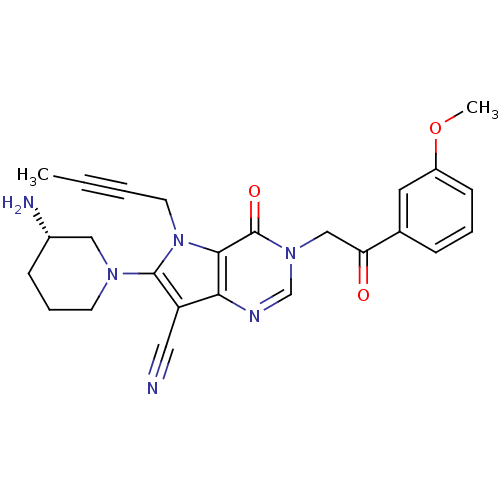
(CHEMBL1951426)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C25H26N6O3/c1-3-4-11-31-23-22(20(13-26)24(31)29-10-6-8-18(27)14-29)28-16-30(25(23)33)15-21(32)17-7-5-9-19(12-17)34-2/h5,7,9,12,16,18H,6,8,10-11,14-15,27H2,1-2H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364164

(CHEMBL1951424)Show SMILES N[C@H]1CCCN(C1)c1c(C#N)c2ncn(Cc3ccnc4ccccc34)c(=O)c2n1Cc1ccccc1 |r| Show InChI InChI=1S/C29H27N7O/c30-15-24-26-27(29(37)35(19-33-26)17-21-12-13-32-25-11-5-4-10-23(21)25)36(16-20-7-2-1-3-8-20)28(24)34-14-6-9-22(31)18-34/h1-5,7-8,10-13,19,22H,6,9,14,16-18,31H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364178
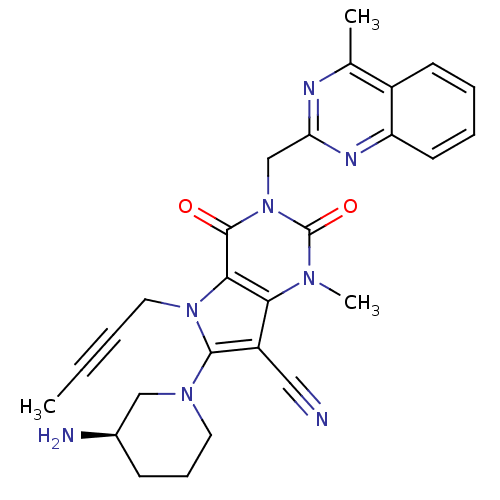
(CHEMBL1951605)Show SMILES CC#CCn1c(N2CCC[C@@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H28N8O2/c1-4-5-13-34-24-23(20(14-28)25(34)33-12-8-9-18(29)15-33)32(3)27(37)35(26(24)36)16-22-30-17(2)19-10-6-7-11-21(19)31-22/h6-7,10-11,18H,8-9,12-13,15-16,29H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364146
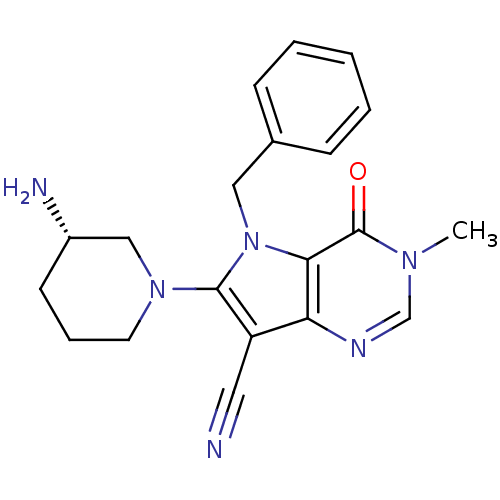
(CHEMBL1951421)Show SMILES Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C20H22N6O/c1-24-13-23-17-16(10-21)19(25-9-5-8-15(22)12-25)26(18(17)20(24)27)11-14-6-3-2-4-7-14/h2-4,6-7,13,15H,5,8-9,11-12,22H2,1H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364144
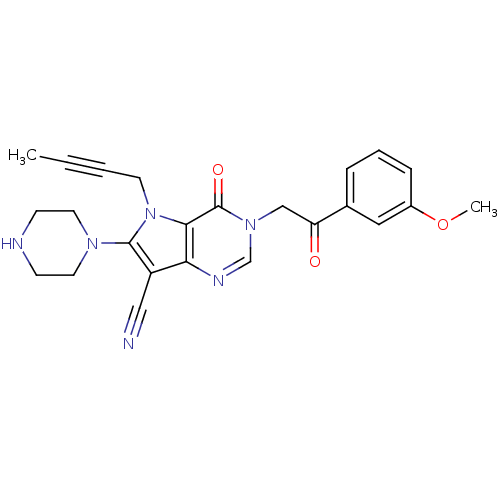
(CHEMBL1951419)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCNCC3)n(CC#CC)c2c1=O Show InChI InChI=1S/C24H24N6O3/c1-3-4-10-30-22-21(19(14-25)23(30)28-11-8-26-9-12-28)27-16-29(24(22)32)15-20(31)17-6-5-7-18(13-17)33-2/h5-7,13,16,26H,8-12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364166

(CHEMBL1951422)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C28H28N6O3/c1-37-22-11-5-9-20(13-22)24(35)17-33-18-31-25-23(14-29)27(32-12-6-10-21(30)16-32)34(26(25)28(33)36)15-19-7-3-2-4-8-19/h2-5,7-9,11,13,18,21H,6,10,12,15-17,30H2,1H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364175

(CHEMBL1951602)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncccc3C#N)c(=O)c12 |r| Show InChI InChI=1S/C25H28N8O2/c1-16(2)8-11-32-22-21(19(13-27)23(32)31-10-5-7-18(28)14-31)30(3)25(35)33(24(22)34)15-20-17(12-26)6-4-9-29-20/h4,6,8-9,18H,5,7,10-11,14-15,28H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364149
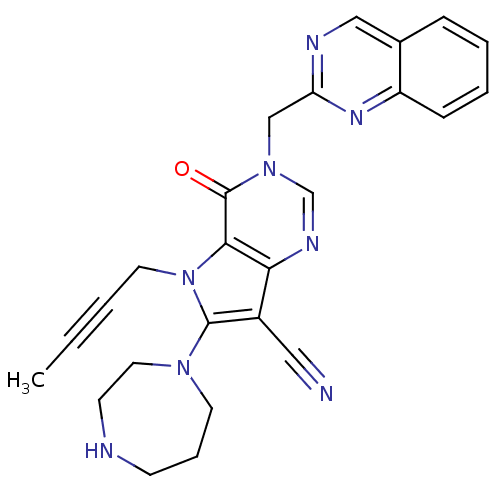
(CHEMBL1951440)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3ncc4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C25H24N8O/c1-2-3-12-33-23-22(19(14-26)24(33)31-11-6-9-27-10-13-31)29-17-32(25(23)34)16-21-28-15-18-7-4-5-8-20(18)30-21/h4-5,7-8,15,17,27H,6,9-13,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364163
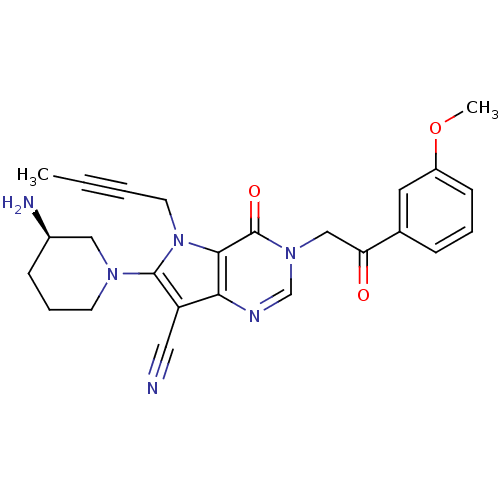
(CHEMBL1951425)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C25H26N6O3/c1-3-4-11-31-23-22(20(13-26)24(31)29-10-6-8-18(27)14-29)28-16-30(25(23)33)15-21(32)17-7-5-9-19(12-17)34-2/h5,7,9,12,16,18H,6,8,10-11,14-15,27H2,1-2H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364151

(CHEMBL1951436)Show SMILES O=c1n(Cc2nccc3ccccc23)cnc2c(C#N)c(N3CCCNCC3)n(Cc3ccccc3)c12 Show InChI InChI=1S/C29H27N7O/c30-17-24-26-27(29(37)35(20-33-26)19-25-23-10-5-4-9-22(23)11-13-32-25)36(18-21-7-2-1-3-8-21)28(24)34-15-6-12-31-14-16-34/h1-5,7-11,13,20,31H,6,12,14-16,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364147

(CHEMBL1951595)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C29H30N6O4/c1-32-25-23(15-30)27(33-13-7-11-21(31)17-33)34(16-19-8-4-3-5-9-19)26(25)28(37)35(29(32)38)18-24(36)20-10-6-12-22(14-20)39-2/h3-6,8-10,12,14,21H,7,11,13,16-18,31H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364180
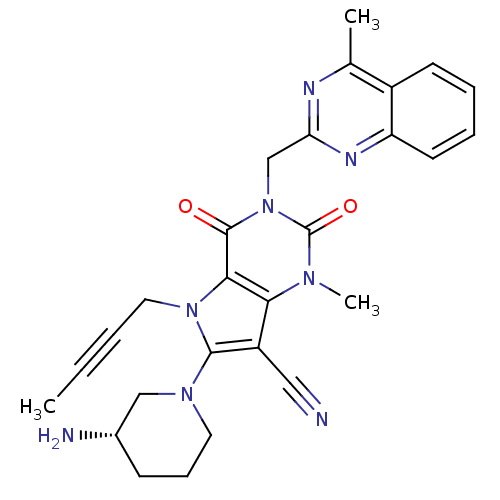
(CHEMBL1951606)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H28N8O2/c1-4-5-13-34-24-23(20(14-28)25(34)33-12-8-9-18(29)15-33)32(3)27(37)35(26(24)36)16-22-30-17(2)19-10-6-7-11-21(19)31-22/h6-7,10-11,18H,8-9,12-13,15-16,29H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364143

(CHEMBL1951418)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCNCC3)n(Cc3ccccc3)c2c1=O Show InChI InChI=1S/C27H26N6O3/c1-36-21-9-5-8-20(14-21)23(34)17-32-18-30-24-22(15-28)26(31-12-10-29-11-13-31)33(25(24)27(32)35)16-19-6-3-2-4-7-19/h2-9,14,18,29H,10-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364185

(CHEMBL1949693)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(22(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-23-20(15-30)17-33-24-8-5-4-7-21(23)24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50364167

(CHEMBL1951600)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C28H32N8O2/c1-17(2)11-13-35-25-24(21(14-29)26(35)34-12-7-8-19(30)15-34)33(4)28(38)36(27(25)37)16-23-31-18(3)20-9-5-6-10-22(20)32-23/h5-6,9-11,19H,7-8,12-13,15-16,30H2,1-4H3/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FAP |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364179

(CHEMBL1951607)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C27H32N6O4/c1-18(2)9-13-32-24-23(21(16-28)25(32)31-12-6-10-29-11-14-31)30(3)27(36)33(26(24)35)17-22(34)19-7-5-8-20(15-19)37-4/h5,7-9,15,29H,6,10-14,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364177

(CHEMBL1951603)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C26H28N6O4/c1-4-5-12-31-23-22(20(14-27)24(31)30-11-7-9-18(28)15-30)29(2)26(35)32(25(23)34)16-21(33)17-8-6-10-19(13-17)36-3/h6,8,10,13,18H,7,9,11-12,15-16,28H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364171

(CHEMBL1951598)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C27H32N6O4/c1-17(2)10-12-32-24-23(21(14-28)25(32)31-11-6-8-19(29)15-31)30(3)27(36)33(26(24)35)16-22(34)18-7-5-9-20(13-18)37-4/h5,7,9-10,13,19H,6,8,11-12,15-16,29H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50364156

(CHEMBL1951432)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-7-20(29)15-32)31-17-33(27(25)35)16-23-21-8-4-3-6-19(21)9-11-30-23/h3-4,6,8-11,17,20H,5,7,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364182

(CHEMBL1951610)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cn4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H30N8O2/c1-18(2)8-13-33-23-22(20(15-27)24(33)31-12-6-9-28-10-14-31)30(3)26(36)34(25(23)35)17-19-16-32-11-5-4-7-21(32)29-19/h4-5,7-8,11,16,28H,6,9-10,12-14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364184

(CHEMBL1951611)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(23(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-24-22(15-30)21-8-5-4-7-20(21)17-33-24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364181

(CHEMBL1951608)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C28H31N7O2/c1-19(2)10-15-34-25-24(22(17-29)26(34)33-14-6-11-30-13-16-33)32(3)28(37)35(27(25)36)18-23-21-8-5-4-7-20(21)9-12-31-23/h4-5,7-10,12,30H,6,11,13-16,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364183

(CHEMBL1951609)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C28H32N8O2/c1-18(2)10-14-35-25-24(21(16-29)26(35)34-13-7-11-30-12-15-34)33(4)28(38)36(27(25)37)17-23-31-19(3)20-8-5-6-9-22(20)32-23/h5-6,8-10,30H,7,11-15,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364142

(CHEMBL1951417)Show InChI InChI=1S/C19H20N6O/c1-23-13-22-16-15(11-20)18(24-9-7-21-8-10-24)25(17(16)19(23)26)12-14-5-3-2-4-6-14/h2-6,13,21H,7-10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364170

(CHEMBL1951596)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(Cc2nccc3ccccc23)c1=O |r| Show InChI InChI=1S/C30H29N7O2/c1-34-26-24(16-31)28(35-15-7-11-22(32)18-35)36(17-20-8-3-2-4-9-20)27(26)29(38)37(30(34)39)19-25-23-12-6-5-10-21(23)13-14-33-25/h2-6,8-10,12-14,22H,7,11,15,17-19,32H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364169

(CHEMBL1951612)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H27N7O2/c1-3-4-15-33-24-23(21(17-28)25(33)32-14-7-11-29-13-16-32)31(2)27(36)34(26(24)35)18-22-20-9-6-5-8-19(20)10-12-30-22/h5-6,8-10,12,29H,7,11,13-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364157

(CHEMBL1951431)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-6-20(29)16-32)31-17-33(27(25)35)15-19-9-11-30-23-8-4-3-7-21(19)23/h3-4,7-11,17,20H,5-6,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364172

(CHEMBL1951597)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H24N6O2/c1-24-17-16(11-22)19(26-10-6-9-15(23)13-26)27(12-14-7-4-3-5-8-14)18(17)20(28)25(2)21(24)29/h3-5,7-8,15H,6,9-10,12-13,23H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364186

(CHEMBL1951614)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(21(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-22-19(15-29)17-32-23-9-6-5-8-20(22)23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50364168

(CHEMBL1951613)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C27H28N8O2/c1-4-5-14-34-24-23(20(16-28)25(34)33-13-8-11-29-12-15-33)32(3)27(37)35(26(24)36)17-22-30-18(2)19-9-6-7-10-21(19)31-22/h6-7,9-10,29H,8,11-15,17H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FAP |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364174

(CHEMBL1951601)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cnc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O2/c1-17(2)10-12-34-24-23(20(13-28)25(34)33-11-6-7-18(29)15-33)32(3)27(37)35(26(24)36)16-19-14-30-21-8-4-5-9-22(21)31-19/h4-5,8-10,14,18H,6-7,11-12,15-16,29H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364167

(CHEMBL1951600)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C28H32N8O2/c1-17(2)11-13-35-25-24(21(14-29)26(35)34-12-7-8-19(30)15-34)33(4)28(38)36(27(25)37)16-23-31-18(3)20-9-5-6-10-22(20)32-23/h5-6,9-11,19H,7-8,12-13,15-16,30H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364173

(CHEMBL1951599)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C28H31N7O2/c1-18(2)11-14-34-25-24(22(15-29)26(34)33-13-6-8-20(30)16-33)32(3)28(37)35(27(25)36)17-23-21-9-5-4-7-19(21)10-12-31-23/h4-5,7,9-12,20H,6,8,13-14,16-17,30H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364171

(CHEMBL1951598)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C27H32N6O4/c1-17(2)10-12-32-24-23(21(14-28)25(32)31-11-6-8-19(29)15-31)30(3)27(36)33(26(24)35)16-22(34)18-7-5-9-20(13-18)37-4/h5,7,9-10,13,19H,6,8,11-12,15-16,29H2,1-4H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364152

(CHEMBL1951437)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H29N7O/c1-19(2)9-14-34-25-24(22(16-28)26(34)32-13-5-10-29-12-15-32)31-18-33(27(25)35)17-23-21-7-4-3-6-20(21)8-11-30-23/h3-4,6-9,11,18,29H,5,10,12-15,17H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364156

(CHEMBL1951432)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-7-20(29)15-32)31-17-33(27(25)35)16-23-21-8-4-3-6-19(21)9-11-30-23/h3-4,6,8-11,17,20H,5,7,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364157

(CHEMBL1951431)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-6-20(29)16-32)31-17-33(27(25)35)15-19-9-11-30-23-8-4-3-7-21(19)23/h3-4,7-11,17,20H,5-6,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364159

(CHEMBL1951429)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364165

(CHEMBL1951423)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C28H28N6O3/c1-37-22-11-5-9-20(13-22)24(35)17-33-18-31-25-23(14-29)27(32-12-6-10-21(30)16-32)34(26(25)28(33)36)15-19-7-3-2-4-8-19/h2-5,7-9,11,13,18,21H,6,10,12,15-17,30H2,1H3/t21-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364168

(CHEMBL1951613)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C27H28N8O2/c1-4-5-14-34-24-23(20(16-28)25(34)33-13-8-11-29-12-15-33)32(3)27(37)35(26(24)36)17-22-30-18(2)19-9-6-7-10-21(19)31-22/h6-7,9-10,29H,8,11-15,17H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364178

(CHEMBL1951605)Show SMILES CC#CCn1c(N2CCC[C@@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H28N8O2/c1-4-5-13-34-24-23(20(14-28)25(34)33-12-8-9-18(29)15-33)32(3)27(37)35(26(24)36)16-22-30-17(2)19-10-6-7-11-21(19)31-22/h6-7,10-11,18H,8-9,12-13,15-16,29H2,1-3H3/t18-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364176

(CHEMBL1951604)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H27N7O2/c1-3-4-14-33-24-23(21(15-28)25(33)32-13-7-9-19(29)16-32)31(2)27(36)34(26(24)35)17-22-20-10-6-5-8-18(20)11-12-30-22/h5-6,8,10-12,19H,7,9,13-14,16-17,29H2,1-2H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364172

(CHEMBL1951597)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H24N6O2/c1-24-17-16(11-22)19(26-10-6-9-15(23)13-26)27(12-14-7-4-3-5-8-14)18(17)20(28)25(2)21(24)29/h3-5,7-8,15H,6,9-10,12-13,23H2,1-2H3/t15-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364170

(CHEMBL1951596)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(Cc2nccc3ccccc23)c1=O |r| Show InChI InChI=1S/C30H29N7O2/c1-34-26-24(16-31)28(35-15-7-11-22(32)18-35)36(17-20-8-3-2-4-9-20)27(26)29(38)37(30(34)39)19-25-23-12-6-5-10-21(23)13-14-33-25/h2-6,8-10,12-14,22H,7,11,15,17-19,32H2,1H3/t22-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364148

(CHEMBL1951439)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H26N8O/c1-3-4-13-34-24-23(20(15-27)25(34)32-12-7-10-28-11-14-32)29-17-33(26(24)35)16-22-30-18(2)19-8-5-6-9-21(19)31-22/h5-6,8-9,17,28H,7,10-14,16H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364160

(CHEMBL1951428)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-8-19(28)15-31)30-17-32(26(24)34)16-22-20-9-5-4-7-18(20)10-11-29-22/h4-5,7,9-11,17,19H,6,8,12-13,15-16,28H2,1H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364146

(CHEMBL1951421)Show SMILES Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C20H22N6O/c1-24-13-23-17-16(10-21)19(25-9-5-8-15(22)12-25)26(18(17)20(24)27)11-14-6-3-2-4-7-14/h2-4,6-7,13,15H,5,8-9,11-12,22H2,1H3/t15-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364169

(CHEMBL1951612)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H27N7O2/c1-3-4-15-33-24-23(21(17-28)25(33)32-14-7-11-29-13-16-32)31(2)27(36)34(26(24)35)18-22-20-9-6-5-8-19(20)10-12-30-22/h5-6,8-10,12,29H,7,11,13-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Qpatch assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364187

(CHEMBL1951416)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(22(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-23-21(15-29)20-9-6-5-8-19(20)17-32-23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364185

(CHEMBL1949693)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(22(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-23-20(15-30)17-33-24-8-5-4-7-21(23)24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364182

(CHEMBL1951610)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cn4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H30N8O2/c1-18(2)8-13-33-23-22(20(15-27)24(33)31-12-6-9-28-10-14-31)30(3)26(36)34(25(23)35)17-19-16-32-11-5-4-7-21(32)29-19/h4-5,7-8,11,16,28H,6,9-10,12-14,17H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364183

(CHEMBL1951609)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C28H32N8O2/c1-18(2)10-14-35-25-24(21(16-29)26(35)34-13-7-11-30-12-15-34)33(4)28(38)36(27(25)37)17-23-31-19(3)20-8-5-6-9-22(20)32-23/h5-6,8-10,30H,7,11-15,17H2,1-4H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364179

(CHEMBL1951607)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C27H32N6O4/c1-18(2)9-13-32-24-23(21(16-28)25(32)31-12-6-10-29-11-14-31)30(3)27(36)33(26(24)35)17-22(34)19-7-5-8-20(15-19)37-4/h5,7-9,15,29H,6,10-14,17H2,1-4H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364167

(CHEMBL1951600)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C28H32N8O2/c1-17(2)11-13-35-25-24(21(14-29)26(35)34-12-7-8-19(30)15-34)33(4)28(38)36(27(25)37)16-23-31-18(3)20-9-5-6-10-22(20)32-23/h5-6,9-11,19H,7-8,12-13,15-16,30H2,1-4H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364153

(CHEMBL1951435)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6])c(=O)c12 |r| Show InChI InChI=1S/C18H24N6O/c1-12(2)6-8-24-16-15(21-11-22(3)18(16)25)14(9-19)17(24)23-7-4-5-13(20)10-23/h6,11,13H,4-5,7-8,10,20H2,1-3H3/t13-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364162

(CHEMBL1951426)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C25H26N6O3/c1-3-4-11-31-23-22(20(13-26)24(31)29-10-6-8-18(27)14-29)28-16-30(25(23)33)15-21(32)17-7-5-9-19(12-17)34-2/h5,7,9,12,16,18H,6,8,10-11,14-15,27H2,1-2H3/t18-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364164

(CHEMBL1951424)Show SMILES N[C@H]1CCCN(C1)c1c(C#N)c2ncn(Cc3ccnc4ccccc34)c(=O)c2n1Cc1ccccc1 |r| Show InChI InChI=1S/C29H27N7O/c30-15-24-26-27(29(37)35(19-33-26)17-21-12-13-32-25-11-5-4-10-23(21)25)36(16-20-7-2-1-3-8-20)28(24)34-14-6-9-22(31)18-34/h1-5,7-8,10-13,19,22H,6,9,14,16-18,31H2/t22-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364144

(CHEMBL1951419)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCNCC3)n(CC#CC)c2c1=O Show InChI InChI=1S/C24H24N6O3/c1-3-4-10-30-22-21(19(14-25)23(30)28-11-8-26-9-12-28)27-16-29(24(22)32)15-20(31)17-6-5-7-18(13-17)33-2/h5-7,13,16,26H,8-12,15H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364181

(CHEMBL1951608)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C28H31N7O2/c1-19(2)10-15-34-25-24(22(17-29)26(34)33-14-6-11-30-13-16-33)32(3)28(37)35(27(25)36)18-23-21-8-5-4-7-20(21)9-12-31-23/h4-5,7-10,12,30H,6,11,13-16,18H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364166

(CHEMBL1951422)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C28H28N6O3/c1-37-22-11-5-9-20(13-22)24(35)17-33-18-31-25-23(14-29)27(32-12-6-10-21(30)16-32)34(26(25)28(33)36)15-19-7-3-2-4-8-19/h2-5,7-9,11,13,18,21H,6,10,12,15-17,30H2,1H3/t21-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364161

(CHEMBL1951427)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-7-19(28)16-31)30-17-32(26(24)34)15-18-10-11-29-22-9-5-4-8-20(18)22/h4-5,8-11,17,19H,6-7,12-13,15-16,28H2,1H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364147

(CHEMBL1951595)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C29H30N6O4/c1-32-25-23(15-30)27(33-13-7-11-21(31)17-33)34(16-19-8-4-3-5-9-19)26(25)28(37)35(29(32)38)18-24(36)20-10-6-12-22(14-20)39-2/h3-6,8-10,12,14,21H,7,11,13,16-18,31H2,1-2H3/t21-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364175

(CHEMBL1951602)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncccc3C#N)c(=O)c12 |r| Show InChI InChI=1S/C25H28N8O2/c1-16(2)8-11-32-22-21(19(13-27)23(32)31-10-5-7-18(28)14-31)30(3)25(35)33(24(22)34)15-20-17(12-26)6-4-9-29-20/h4,6,8-9,18H,5,7,10-11,14-15,28H2,1-3H3/t18-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364155

(CHEMBL1951433)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O/c1-17(2)10-12-35-25-24(21(13-28)26(35)33-11-6-7-19(29)14-33)30-16-34(27(25)36)15-23-31-18(3)20-8-4-5-9-22(20)32-23/h4-5,8-10,16,19H,6-7,11-12,14-15,29H2,1-3H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364149

(CHEMBL1951440)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3ncc4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C25H24N8O/c1-2-3-12-33-23-22(19(14-26)24(33)31-11-6-9-27-10-13-31)29-17-32(25(23)34)16-21-28-15-18-7-4-5-8-20(18)30-21/h4-5,7-8,15,17,27H,6,9-13,16H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364145

(CHEMBL1951420)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C25H28N6O3/c1-17(2)7-10-31-23-22(20(14-26)24(31)29-11-8-27-9-12-29)28-16-30(25(23)33)15-21(32)18-5-4-6-19(13-18)34-3/h4-7,13,16,27H,8-12,15H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364174

(CHEMBL1951601)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cnc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O2/c1-17(2)10-12-34-24-23(20(13-28)25(34)33-11-6-7-18(29)15-33)32(3)27(37)35(26(24)36)16-19-14-30-21-8-4-5-9-22(21)31-19/h4-5,8-10,14,18H,6-7,11-12,15-16,29H2,1-3H3/t18-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364169

(CHEMBL1951612)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H27N7O2/c1-3-4-15-33-24-23(21(17-28)25(33)32-14-7-11-29-13-16-32)31(2)27(36)34(26(24)35)18-22-20-9-6-5-8-19(20)10-12-30-22/h5-6,8-10,12,29H,7,11,13-16,18H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364163

(CHEMBL1951425)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C25H26N6O3/c1-3-4-11-31-23-22(20(13-26)24(31)29-10-6-8-18(27)14-29)28-16-30(25(23)33)15-21(32)17-7-5-9-19(12-17)34-2/h5,7,9,12,16,18H,6,8,10-11,14-15,27H2,1-2H3/t18-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364154

(CHEMBL1951434)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ncc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C26H28N8O/c1-17(2)9-11-34-24-23(20(12-27)25(34)32-10-5-7-19(28)14-32)30-16-33(26(24)35)15-22-29-13-18-6-3-4-8-21(18)31-22/h3-4,6,8-9,13,16,19H,5,7,10-11,14-15,28H2,1-2H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364151

(CHEMBL1951436)Show SMILES O=c1n(Cc2nccc3ccccc23)cnc2c(C#N)c(N3CCCNCC3)n(Cc3ccccc3)c12 Show InChI InChI=1S/C29H27N7O/c30-17-24-26-27(29(37)35(20-33-26)19-25-23-10-5-4-9-22(23)11-13-32-25)36(18-21-7-2-1-3-8-21)28(24)34-15-6-12-31-14-16-34/h1-5,7-11,13,20,31H,6,12,14-16,18-19H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364150

(CHEMBL1951438)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C26H25N7O/c1-2-3-14-33-24-23(21(16-27)25(33)31-13-6-10-28-12-15-31)30-18-32(26(24)34)17-22-20-8-5-4-7-19(20)9-11-29-22/h4-5,7-9,11,18,28H,6,10,12-15,17H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364186

(CHEMBL1951614)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(21(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-22-19(15-29)17-32-23-9-6-5-8-20(22)23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364180

(CHEMBL1951606)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H28N8O2/c1-4-5-13-34-24-23(20(14-28)25(34)33-12-8-9-18(29)15-33)32(3)27(37)35(26(24)36)16-22-30-17(2)19-10-6-7-11-21(19)31-22/h6-7,10-11,18H,8-9,12-13,15-16,29H2,1-3H3/t18-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364158

(CHEMBL1951430)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364143

(CHEMBL1951418)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCNCC3)n(Cc3ccccc3)c2c1=O Show InChI InChI=1S/C27H26N6O3/c1-36-21-9-5-8-20(14-21)23(34)17-32-18-30-24-22(15-28)26(31-12-10-29-11-13-31)33(25(24)27(32)35)16-19-6-3-2-4-7-19/h2-9,14,18,29H,10-13,16-17H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364142

(CHEMBL1951417)Show InChI InChI=1S/C19H20N6O/c1-23-13-22-16-15(11-20)18(24-9-7-21-8-10-24)25(17(16)19(23)26)12-14-5-3-2-4-6-14/h2-6,13,21H,7-10,12H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364184

(CHEMBL1951611)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(23(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-24-22(15-30)21-8-5-4-7-20(21)17-33-24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364177

(CHEMBL1951603)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C26H28N6O4/c1-4-5-12-31-23-22(20(14-27)24(31)30-11-7-9-18(28)15-30)29(2)26(35)32(25(23)34)16-21(33)17-8-6-10-19(13-17)36-3/h6,8,10,13,18H,7,9,11-12,15-16,28H2,1-3H3/t18-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50364173

(CHEMBL1951599)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C28H31N7O2/c1-18(2)11-14-34-25-24(22(15-29)26(34)33-13-6-8-20(30)16-33)32(3)28(37)35(27(25)36)17-23-21-9-5-4-7-19(21)10-12-31-23/h4-5,7,9-12,20H,6,8,13-14,16-17,30H2,1-3H3/t20-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of M1 receptor |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364187

(CHEMBL1951416)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(22(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-23-21(15-29)20-9-6-5-8-19(20)17-32-23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364168

(CHEMBL1951613)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C27H28N8O2/c1-4-5-14-34-24-23(20(16-28)25(34)33-13-8-11-29-12-15-33)32(3)27(37)35(26(24)36)17-22-30-18(2)19-9-6-7-10-21(19)31-22/h6-7,9-10,29H,8,11-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364175

(CHEMBL1951602)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncccc3C#N)c(=O)c12 |r| Show InChI InChI=1S/C25H28N8O2/c1-16(2)8-11-32-22-21(19(13-27)23(32)31-10-5-7-18(28)14-31)30(3)25(35)33(24(22)34)15-20-17(12-26)6-4-9-29-20/h4,6,8-9,18H,5,7,10-11,14-15,28H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364178

(CHEMBL1951605)Show SMILES CC#CCn1c(N2CCC[C@@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H28N8O2/c1-4-5-13-34-24-23(20(14-28)25(34)33-12-8-9-18(29)15-33)32(3)27(37)35(26(24)36)16-22-30-17(2)19-10-6-7-11-21(19)31-22/h6-7,10-11,18H,8-9,12-13,15-16,29H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364176

(CHEMBL1951604)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H27N7O2/c1-3-4-14-33-24-23(21(15-28)25(33)32-13-7-9-19(29)16-32)31(2)27(36)34(26(24)35)17-22-20-10-6-5-8-18(20)11-12-30-22/h5-6,8,10-12,19H,7,9,13-14,16-17,29H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364180

(CHEMBL1951606)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H28N8O2/c1-4-5-13-34-24-23(20(14-28)25(34)33-12-8-9-18(29)15-33)32(3)27(37)35(26(24)36)16-22-30-17(2)19-10-6-7-11-21(19)31-22/h6-7,10-11,18H,8-9,12-13,15-16,29H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data