

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50382632 (CHEMBL2023529) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50382631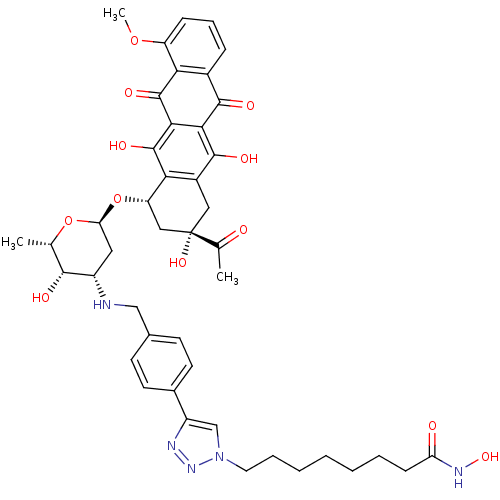 (CHEMBL2023530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50382631 (CHEMBL2023530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50382632 (CHEMBL2023529) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50382634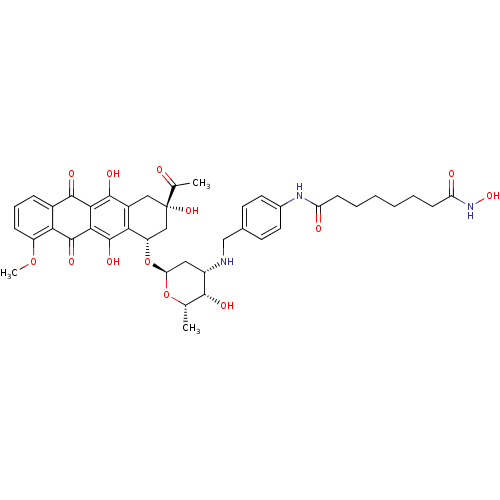 (CHEMBL2023526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50382633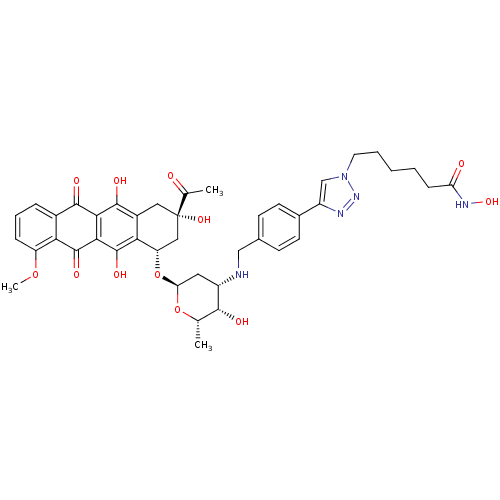 (CHEMBL2023528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50382634 (CHEMBL2023526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50382633 (CHEMBL2023528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50382634 (CHEMBL2023526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50382631 (CHEMBL2023530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50382635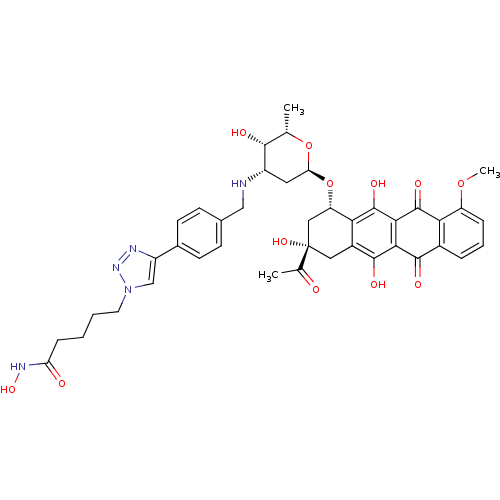 (CHEMBL2023527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 555 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50382632 (CHEMBL2023529) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50382633 (CHEMBL2023528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC8 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50382635 (CHEMBL2023527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 in human HeLa cell nuclear extract using Fluor de Lys as substrate after 15 mins by fluorometric analysis | J Med Chem 55: 1465-77 (2012) Article DOI: 10.1021/jm200799p BindingDB Entry DOI: 10.7270/Q24F1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||