 Found 55 hits of Enzyme Inhibition Constant Data
Found 55 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364748
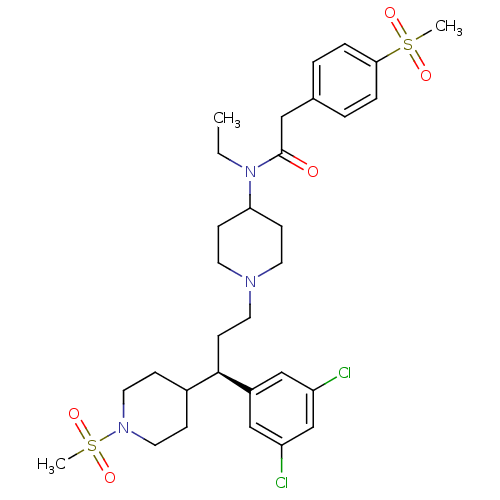
(CHEMBL1952100)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(Cl)cc(Cl)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43Cl2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364751
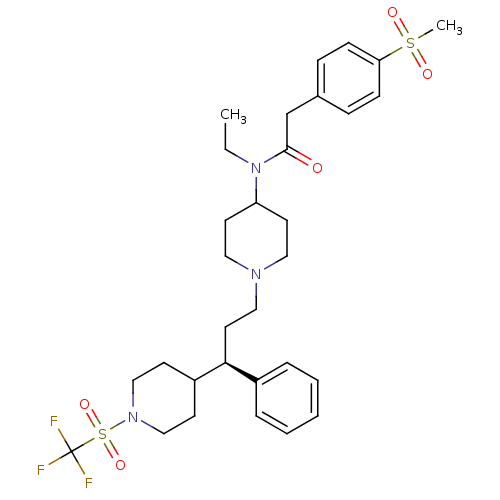
(CHEMBL1951908)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(=O)(=O)C(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H42F3N3O5S2/c1-3-37(30(38)23-24-9-11-28(12-10-24)43(2,39)40)27-15-18-35(19-16-27)20-17-29(25-7-5-4-6-8-25)26-13-21-36(22-14-26)44(41,42)31(32,33)34/h4-12,26-27,29H,3,13-23H2,1-2H3/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364745
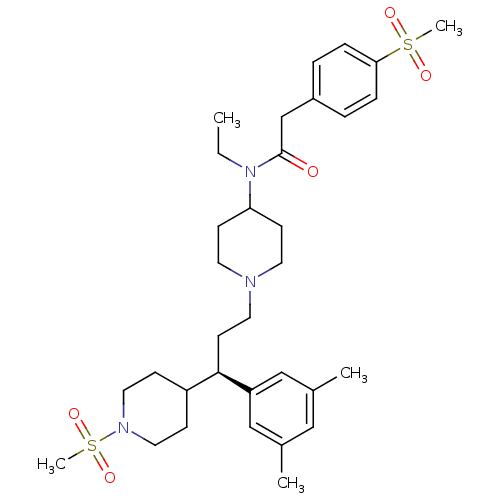
(CHEMBL1951916)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(C)cc(C)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C33H49N3O5S2/c1-6-36(33(37)24-27-7-9-31(10-8-27)42(4,38)39)30-13-16-34(17-14-30)18-15-32(29-22-25(2)21-26(3)23-29)28-11-19-35(20-12-28)43(5,40)41/h7-10,21-23,28,30,32H,6,11-20,24H2,1-5H3/t32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364743
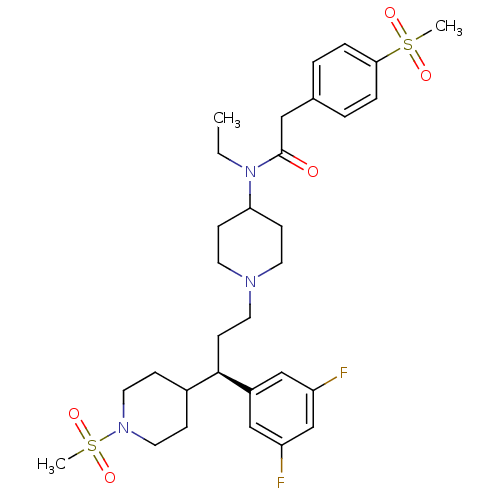
(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50185666
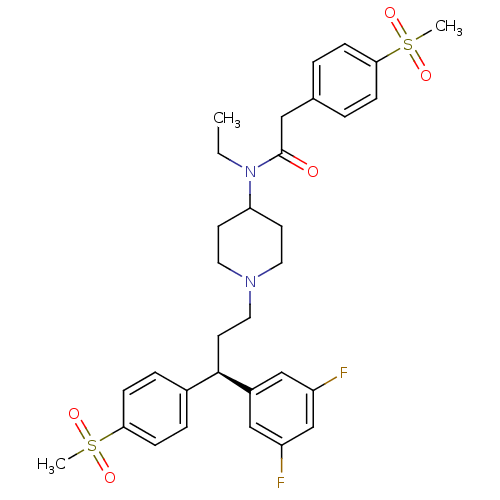
((R)-N-(1-(3-(3,5-difluorophenyl)-3-(4-(methylsulfo...)Show SMILES CCN(C1CCN(CC[C@H](c2ccc(cc2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H38F2N2O5S2/c1-4-36(32(37)19-23-5-9-29(10-6-23)42(2,38)39)28-13-16-35(17-14-28)18-15-31(25-20-26(33)22-27(34)21-25)24-7-11-30(12-8-24)43(3,40)41/h5-12,20-22,28,31H,4,13-19H2,1-3H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364771
(CHEMBL1951913)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(C)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C32H47N3O5S2/c1-5-35(32(36)24-26-9-11-30(12-10-26)41(3,37)38)29-15-18-33(19-16-29)20-17-31(28-8-6-7-25(2)23-28)27-13-21-34(22-14-27)42(4,39)40/h6-12,23,27,29,31H,5,13-22,24H2,1-4H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364746
(CHEMBL1951917)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(Cl)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H44ClN3O5S2/c1-4-35(31(36)22-24-8-10-29(11-9-24)41(2,37)38)28-14-17-33(18-15-28)19-16-30(26-6-5-7-27(32)23-26)25-12-20-34(21-13-25)42(3,39)40/h5-11,23,25,28,30H,4,12-22H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364770

(CHEMBL1951912)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H44FN3O5S2/c1-4-35(31(36)22-24-8-10-29(11-9-24)41(2,37)38)28-14-17-33(18-15-28)19-16-30(26-6-5-7-27(32)23-26)25-12-20-34(21-13-25)42(3,39)40/h5-11,23,25,28,30H,4,12-22H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in allo T cells assessed as inhibition of MIP-1beta-induced chemotaxis |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364744
(CHEMBL1951915)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(Cl)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43ClFN3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364750

(CHEMBL1951906)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H45N3O5S2/c1-4-34(31(35)24-25-10-12-29(13-11-25)40(2,36)37)28-16-19-32(20-17-28)21-18-30(26-8-6-5-7-9-26)27-14-22-33(23-15-27)41(3,38)39/h5-13,27-28,30H,4,14-24H2,1-3H3/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364752
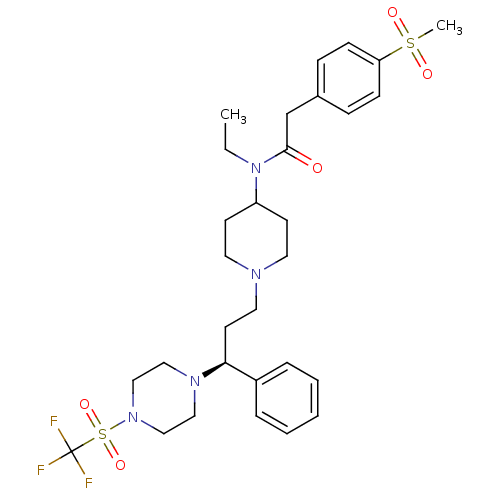
(CHEMBL1951898)Show SMILES CCN(C1CCN(CC[C@H](N2CCN(CC2)S(=O)(=O)C(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C30H41F3N4O5S2/c1-3-37(29(38)23-24-9-11-27(12-10-24)43(2,39)40)26-13-16-34(17-14-26)18-15-28(25-7-5-4-6-8-25)35-19-21-36(22-20-35)44(41,42)30(31,32)33/h4-12,26,28H,3,13-23H2,1-2H3/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364766

(CHEMBL1951907)Show SMILES CCN(C1CCN(CCC(C2CCN(CC2)S(=O)(=O)CC)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H47N3O5S2/c1-4-35(32(36)25-26-11-13-30(14-12-26)41(3,37)38)29-17-20-33(21-18-29)22-19-31(27-9-7-6-8-10-27)28-15-23-34(24-16-28)42(39,40)5-2/h6-14,28-29,31H,4-5,15-25H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364756

(CHEMBL1951894)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)c2cccc(F)c2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C35H45FN4O3S/c1-3-40(35(41)26-28-12-14-33(15-13-28)44(2,42)43)31-16-19-37(20-17-31)21-18-34(29-8-5-4-6-9-29)39-24-22-38(23-25-39)32-11-7-10-30(36)27-32/h4-15,27,31,34H,3,16-26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364747
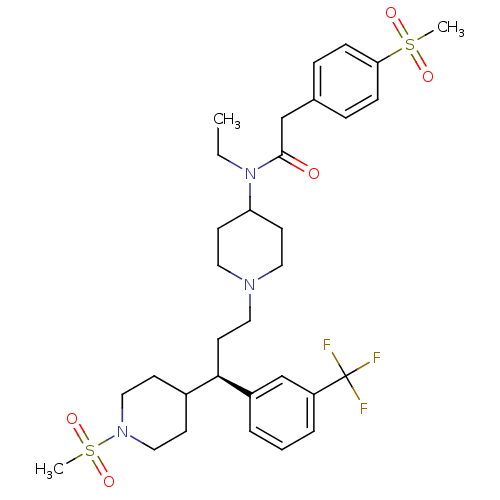
(CHEMBL1951918)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(c2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C32H44F3N3O5S2/c1-4-38(31(39)22-24-8-10-29(11-9-24)44(2,40)41)28-14-17-36(18-15-28)19-16-30(25-12-20-37(21-13-25)45(3,42)43)26-6-5-7-27(23-26)32(33,34)35/h5-11,23,25,28,30H,4,12-22H2,1-3H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364769

(CHEMBL1951911)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(OC)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C32H47N3O6S2/c1-5-35(32(36)23-25-9-11-30(12-10-25)42(3,37)38)28-15-18-33(19-16-28)20-17-31(27-7-6-8-29(24-27)41-2)26-13-21-34(22-14-26)43(4,39)40/h6-12,24,26,28,31H,5,13-23H2,1-4H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364755

(CHEMBL1951893)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)c2ccccc2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C35H46N4O3S/c1-3-39(35(40)28-29-14-16-33(17-15-29)43(2,41)42)32-18-21-36(22-19-32)23-20-34(30-10-6-4-7-11-30)38-26-24-37(25-27-38)31-12-8-5-9-13-31/h4-17,32,34H,3,18-28H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364767

(CHEMBL1949696)Show SMILES CCN(C1CCN(CCC(C2CCN(CC2)S(=O)(=O)CC(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H44F3N3O5S2/c1-3-38(31(39)23-25-9-11-29(12-10-25)44(2,40)41)28-15-18-36(19-16-28)20-17-30(26-7-5-4-6-8-26)27-13-21-37(22-14-27)45(42,43)24-32(33,34)35/h4-12,27-28,30H,3,13-24H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364764
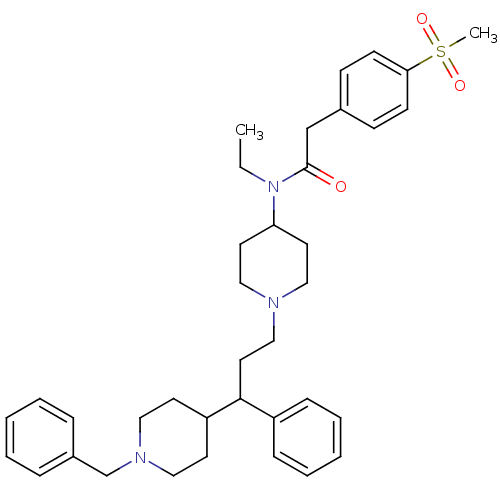
(CHEMBL1951904)Show SMILES CCN(C1CCN(CCC(C2CCN(Cc3ccccc3)CC2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C37H49N3O3S/c1-3-40(37(41)28-30-14-16-35(17-15-30)44(2,42)43)34-20-25-38(26-21-34)27-22-36(32-12-8-5-9-13-32)33-18-23-39(24-19-33)29-31-10-6-4-7-11-31/h4-17,33-34,36H,3,18-29H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364753

(CHEMBL1951899)Show SMILES CCN(C1CCN(CC[C@H](N2CCN(CC2)S(=O)(=O)CC(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F3N4O5S2/c1-3-38(30(39)23-25-9-11-28(12-10-25)44(2,40)41)27-13-16-35(17-14-27)18-15-29(26-7-5-4-6-8-26)36-19-21-37(22-20-36)45(42,43)24-31(32,33)34/h4-12,27,29H,3,13-24H2,1-2H3/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364759

(CHEMBL1951897)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)S(=O)(=O)CC)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H46N4O5S2/c1-4-35(31(36)25-26-11-13-29(14-12-26)41(3,37)38)28-15-18-32(19-16-28)20-17-30(27-9-7-6-8-10-27)33-21-23-34(24-22-33)42(39,40)5-2/h6-14,28,30H,4-5,15-25H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364749
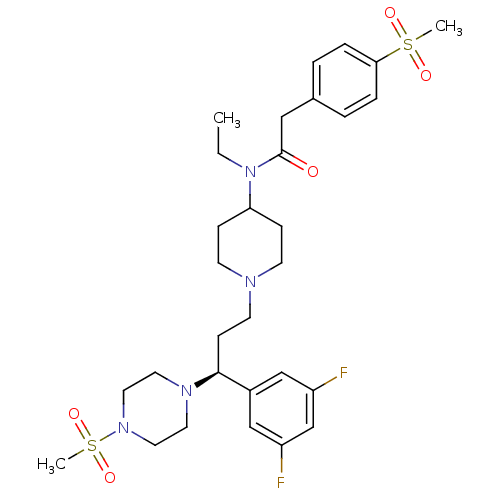
(CHEMBL1951909)Show SMILES CCN(C1CCN(CC[C@H](N2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C30H42F2N4O5S2/c1-4-36(30(37)19-23-5-7-28(8-6-23)42(2,38)39)27-9-12-33(13-10-27)14-11-29(24-20-25(31)22-26(32)21-24)34-15-17-35(18-16-34)43(3,40)41/h5-8,20-22,27,29H,4,9-19H2,1-3H3/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364765
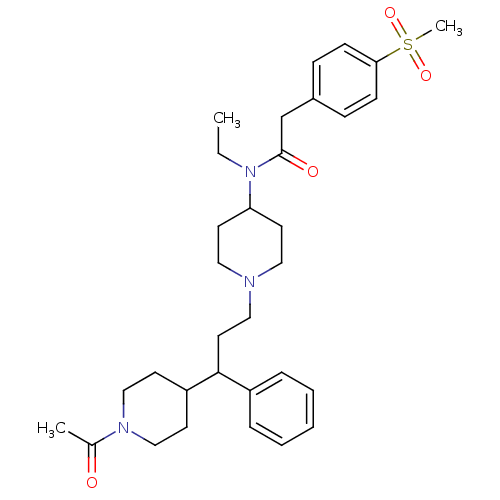
(CHEMBL1951905)Show SMILES CCN(C1CCN(CCC(C2CCN(CC2)C(C)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H45N3O4S/c1-4-35(32(37)24-26-10-12-30(13-11-26)40(3,38)39)29-16-19-33(20-17-29)21-18-31(27-8-6-5-7-9-27)28-14-22-34(23-15-28)25(2)36/h5-13,28-29,31H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364758
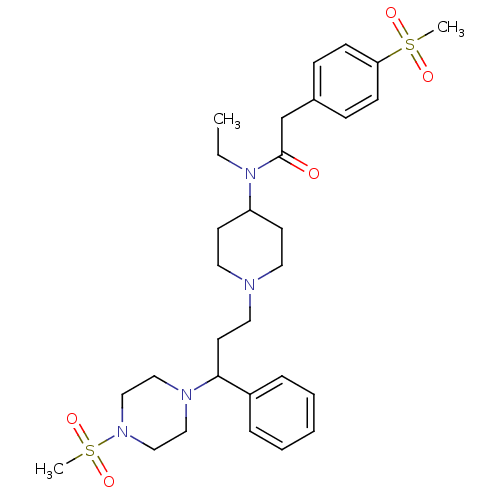
(CHEMBL1951896)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)S(C)(=O)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C30H44N4O5S2/c1-4-34(30(35)24-25-10-12-28(13-11-25)40(2,36)37)27-14-17-31(18-15-27)19-16-29(26-8-6-5-7-9-26)32-20-22-33(23-21-32)41(3,38)39/h5-13,27,29H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364762

(CHEMBL1951902)Show SMILES CCN(C1CCN(CCC(N2CCC(CC2)C(C)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H45N3O4S/c1-4-35(32(37)24-26-10-12-30(13-11-26)40(3,38)39)29-16-19-33(20-17-29)21-18-31(28-8-6-5-7-9-28)34-22-14-27(15-23-34)25(2)36/h5-13,27,29,31H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364763

(CHEMBL1951903)Show SMILES CCN(C1CCN(CCC(C2CCN(C)CC2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H45N3O3S/c1-4-34(31(35)24-25-10-12-29(13-11-25)38(3,36)37)28-16-21-33(22-17-28)23-18-30(26-8-6-5-7-9-26)27-14-19-32(2)20-15-27/h5-13,27-28,30H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364761
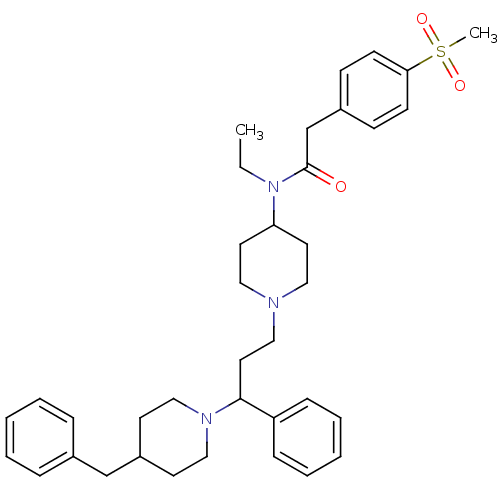
(CHEMBL1951901)Show SMILES CCN(C1CCN(CCC(N2CCC(Cc3ccccc3)CC2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C37H49N3O3S/c1-3-40(37(41)29-31-14-16-35(17-15-31)44(2,42)43)34-20-23-38(24-21-34)25-22-36(33-12-8-5-9-13-33)39-26-18-32(19-27-39)28-30-10-6-4-7-11-30/h4-17,32,34,36H,3,18-29H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364757

(CHEMBL1951895)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)C(C)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H44N4O4S/c1-4-35(31(37)24-26-10-12-29(13-11-26)40(3,38)39)28-14-17-32(18-15-28)19-16-30(27-8-6-5-7-9-27)34-22-20-33(21-23-34)25(2)36/h5-13,28,30H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364760

(CHEMBL1951900)Show SMILES CCN(C1CCN(CCC(N2CCC(C)CC2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H45N3O3S/c1-4-34(31(35)24-26-10-12-29(13-11-26)38(3,36)37)28-16-19-32(20-17-28)21-18-30(27-8-6-5-7-9-27)33-22-14-25(2)15-23-33/h5-13,25,28,30H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364754
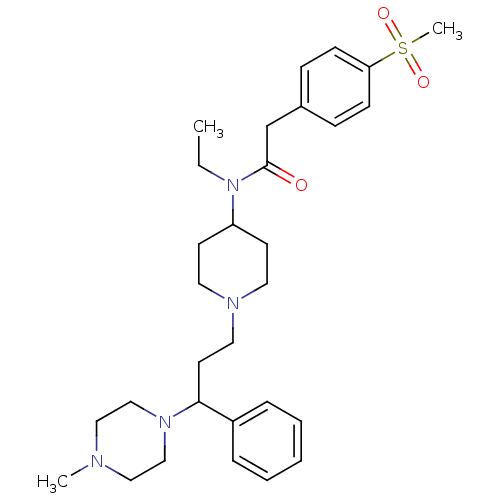
(CHEMBL1951892)Show SMILES CCN(C1CCN(CCC(N2CCN(C)CC2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C30H44N4O3S/c1-4-34(30(35)24-25-10-12-28(13-11-25)38(3,36)37)27-14-17-32(18-15-27)19-16-29(26-8-6-5-7-9-26)33-22-20-31(2)21-23-33/h5-13,27,29H,4,14-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364768

(CHEMBL1951910)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(c2)S(C)(=O)=O)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C32H47N3O7S3/c1-5-35(32(36)23-25-9-11-29(12-10-25)43(2,37)38)28-15-18-33(19-16-28)20-17-31(26-13-21-34(22-14-26)45(4,41)42)27-7-6-8-30(24-27)44(3,39)40/h6-12,24,26,28,31H,5,13-23H2,1-4H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364751

(CHEMBL1951908)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(=O)(=O)C(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H42F3N3O5S2/c1-3-37(30(38)23-24-9-11-28(12-10-24)43(2,39)40)27-15-18-35(19-16-27)20-17-29(25-7-5-4-6-8-25)26-13-21-36(22-14-26)44(41,42)31(32,33)34/h4-12,26-27,29H,3,13-23H2,1-2H3/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185666

((R)-N-(1-(3-(3,5-difluorophenyl)-3-(4-(methylsulfo...)Show SMILES CCN(C1CCN(CC[C@H](c2ccc(cc2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H38F2N2O5S2/c1-4-36(32(37)19-23-5-9-29(10-6-23)42(2,38)39)28-13-16-35(17-14-28)18-15-31(25-20-26(33)22-27(34)21-25)24-7-11-30(12-8-24)43(3,40)41/h5-12,20-22,28,31H,4,13-19H2,1-3H3/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by electrophysiological assay |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364756

(CHEMBL1951894)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)c2cccc(F)c2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C35H45FN4O3S/c1-3-40(35(41)26-28-12-14-33(15-13-28)44(2,42)43)31-16-19-37(20-17-31)21-18-34(29-8-5-4-6-9-29)39-24-22-38(23-25-39)32-11-7-10-30(36)27-32/h4-15,27,31,34H,3,16-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364752

(CHEMBL1951898)Show SMILES CCN(C1CCN(CC[C@H](N2CCN(CC2)S(=O)(=O)C(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C30H41F3N4O5S2/c1-3-37(29(38)23-24-9-11-27(12-10-24)43(2,39)40)26-13-16-34(17-14-26)18-15-28(25-7-5-4-6-8-25)35-19-21-36(22-20-35)44(41,42)30(31,32)33/h4-12,26,28H,3,13-23H2,1-2H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50364749

(CHEMBL1951909)Show SMILES CCN(C1CCN(CC[C@H](N2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C30H42F2N4O5S2/c1-4-36(30(37)19-23-5-7-28(8-6-23)42(2,38)39)27-9-12-33(13-10-27)14-11-29(24-20-25(31)22-26(32)21-24)34-15-17-35(18-16-34)43(3,40)41/h5-8,20-22,27,29H,4,9-19H2,1-3H3/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR5 expressed in CHO cells |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364745

(CHEMBL1951916)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(C)cc(C)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C33H49N3O5S2/c1-6-36(33(37)24-27-7-9-31(10-8-27)42(4,38)39)30-13-16-34(17-14-30)18-15-32(29-22-25(2)21-26(3)23-29)28-11-19-35(20-12-28)43(5,40)41/h7-10,21-23,28,30,32H,6,11-20,24H2,1-5H3/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364753

(CHEMBL1951899)Show SMILES CCN(C1CCN(CC[C@H](N2CCN(CC2)S(=O)(=O)CC(F)(F)F)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F3N4O5S2/c1-3-38(30(39)23-25-9-11-28(12-10-25)44(2,40)41)27-13-16-35(17-14-27)18-15-29(26-7-5-4-6-8-26)36-19-21-37(22-20-36)45(42,43)24-31(32,33)34/h4-12,27,29H,3,13-24H2,1-2H3/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364748

(CHEMBL1952100)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(Cl)cc(Cl)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43Cl2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185666

((R)-N-(1-(3-(3,5-difluorophenyl)-3-(4-(methylsulfo...)Show SMILES CCN(C1CCN(CC[C@H](c2ccc(cc2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H38F2N2O5S2/c1-4-36(32(37)19-23-5-9-29(10-6-23)42(2,38)39)28-13-16-35(17-14-28)18-15-31(25-20-26(33)22-27(34)21-25)24-7-11-30(12-8-24)43(3,40)41/h5-12,20-22,28,31H,4,13-19H2,1-3H3/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364747

(CHEMBL1951918)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(c2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C32H44F3N3O5S2/c1-4-38(31(39)22-24-8-10-29(11-9-24)44(2,40)41)28-14-17-36(18-15-28)19-16-30(25-12-20-37(21-13-25)45(3,42)43)26-6-5-7-27(23-26)32(33,34)35/h5-11,23,25,28,30H,4,12-22H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364746

(CHEMBL1951917)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(Cl)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H44ClN3O5S2/c1-4-35(31(36)22-24-8-10-29(11-9-24)41(2,37)38)28-14-17-33(18-15-28)19-16-30(26-6-5-7-27(32)23-26)25-12-20-34(21-13-25)42(3,39)40/h5-11,23,25,28,30H,4,12-22H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by electrophysiological assay |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364755

(CHEMBL1951893)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)c2ccccc2)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C35H46N4O3S/c1-3-39(35(40)28-29-14-16-33(17-15-29)43(2,41)42)32-18-21-36(22-19-32)23-20-34(30-10-6-4-7-11-30)38-26-24-37(25-27-38)31-12-8-5-9-13-31/h4-17,32,34H,3,18-28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364744

(CHEMBL1951915)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(Cl)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43ClFN3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364758

(CHEMBL1951896)Show SMILES CCN(C1CCN(CCC(N2CCN(CC2)S(C)(=O)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C30H44N4O5S2/c1-4-34(30(35)24-25-10-12-28(13-11-25)40(2,36)37)27-14-17-31(18-15-27)19-16-29(26-8-6-5-7-9-26)32-20-22-33(23-21-32)41(3,38)39/h5-13,27,29H,4,14-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364750

(CHEMBL1951906)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2ccccc2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H45N3O5S2/c1-4-34(31(35)24-25-10-12-29(13-11-25)40(2,36)37)28-16-19-32(20-17-28)21-18-30(26-8-6-5-7-9-26)27-14-22-33(23-15-27)41(3,38)39/h5-13,27-28,30H,4,14-24H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364769

(CHEMBL1951911)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(OC)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C32H47N3O6S2/c1-5-35(32(36)23-25-9-11-30(12-10-25)42(3,37)38)28-15-18-33(19-16-28)20-17-31(27-7-6-8-29(24-27)41-2)26-13-21-34(22-14-26)43(4,39)40/h6-12,24,26,28,31H,5,13-23H2,1-4H3/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364770

(CHEMBL1951912)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cccc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H44FN3O5S2/c1-4-35(31(36)22-24-8-10-29(11-9-24)41(2,37)38)28-14-17-33(18-15-28)19-16-30(26-6-5-7-27(32)23-26)25-12-20-34(21-13-25)42(3,39)40/h5-11,23,25,28,30H,4,12-22H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of 3,7-Bis[2-(4-nitro[3,5-3H]phenyl)ethyl]-3,7-diazabicyclo[3.3.1]nonane from human ERG expressed in HEK cells after 3 hrs |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50364743

(CHEMBL1951914)Show SMILES CCN(C1CCN(CC[C@H](C2CCN(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C31H43F2N3O5S2/c1-4-36(31(37)19-23-5-7-29(8-6-23)42(2,38)39)28-11-14-34(15-12-28)16-13-30(25-20-26(32)22-27(33)21-25)24-9-17-35(18-10-24)43(3,40)41/h5-8,20-22,24,28,30H,4,9-19H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A1 |
Bioorg Med Chem Lett 22: 1655-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.117
BindingDB Entry DOI: 10.7270/Q25H7GQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data