 Found 82 hits of Enzyme Inhibition Constant Data
Found 82 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364921
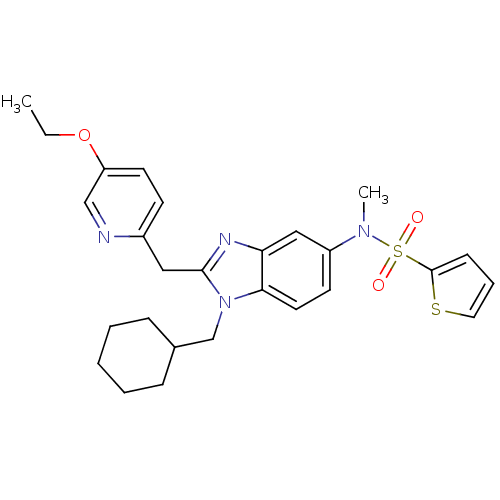
(CHEMBL1950329)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCCC2)N(C)S(=O)(=O)c2cccs2)nc1 Show InChI InChI=1S/C27H32N4O3S2/c1-3-34-23-13-11-21(28-18-23)16-26-29-24-17-22(30(2)36(32,33)27-10-7-15-35-27)12-14-25(24)31(26)19-20-8-5-4-6-9-20/h7,10-15,17-18,20H,3-6,8-9,16,19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364921

(CHEMBL1950329)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCCC2)N(C)S(=O)(=O)c2cccs2)nc1 Show InChI InChI=1S/C27H32N4O3S2/c1-3-34-23-13-11-21(28-18-23)16-26-29-24-17-22(30(2)36(32,33)27-10-7-15-35-27)12-14-25(24)31(26)19-20-8-5-4-6-9-20/h7,10-15,17-18,20H,3-6,8-9,16,19H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364920
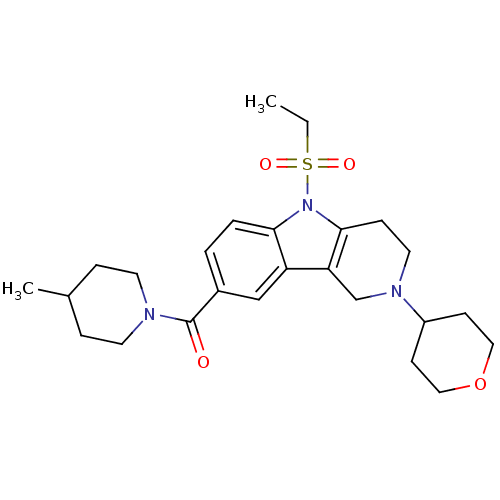
(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364923
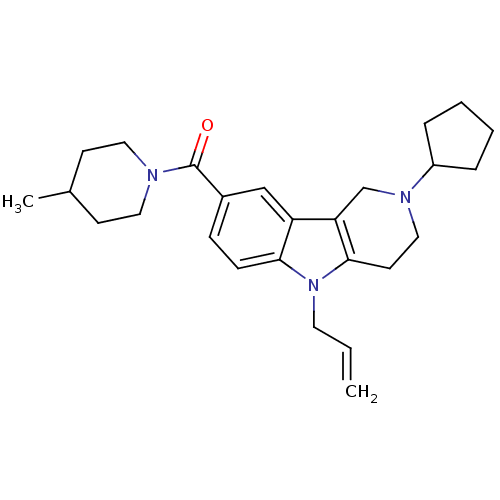
(CHEMBL1950333)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C26H35N3O/c1-3-13-29-24-9-8-20(26(30)27-14-10-19(2)11-15-27)17-22(24)23-18-28(16-12-25(23)29)21-6-4-5-7-21/h3,8-9,17,19,21H,1,4-7,10-16,18H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364922
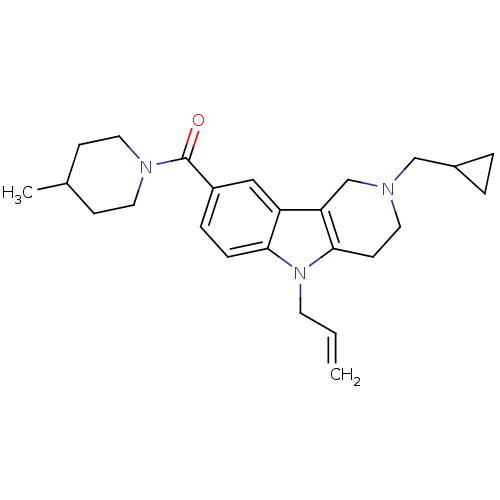
(CHEMBL1950330)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(CC4CC4)Cc3c2c1 Show InChI InChI=1S/C25H33N3O/c1-3-11-28-23-7-6-20(25(29)27-13-8-18(2)9-14-27)15-21(23)22-17-26(12-10-24(22)28)16-19-4-5-19/h3,6-7,15,18-19H,1,4-5,8-14,16-17H2,2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364925

(CHEMBL1950340)Show SMILES CCCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C26H37N3O/c1-3-13-29-24-9-8-20(26(30)27-14-10-19(2)11-15-27)17-22(24)23-18-28(16-12-25(23)29)21-6-4-5-7-21/h8-9,17,19,21H,3-7,10-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364935

(CHEMBL1950350)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C25H35N3O3S/c1-3-32(30,31)28-23-9-8-19(25(29)26-13-10-18(2)11-14-26)16-21(23)22-17-27(15-12-24(22)28)20-6-4-5-7-20/h8-9,16,18,20H,3-7,10-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50218116
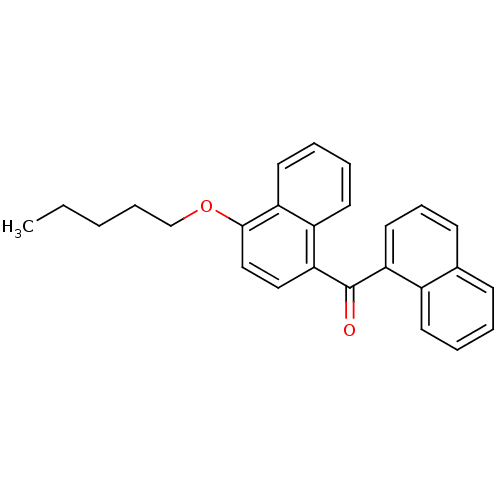
(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364932

(CHEMBL1950347)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C26H37N3O3S/c1-3-16-33(31,32)29-24-9-8-20(26(30)27-13-10-19(2)11-14-27)17-22(24)23-18-28(15-12-25(23)29)21-6-4-5-7-21/h8-9,17,19,21H,3-7,10-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364938
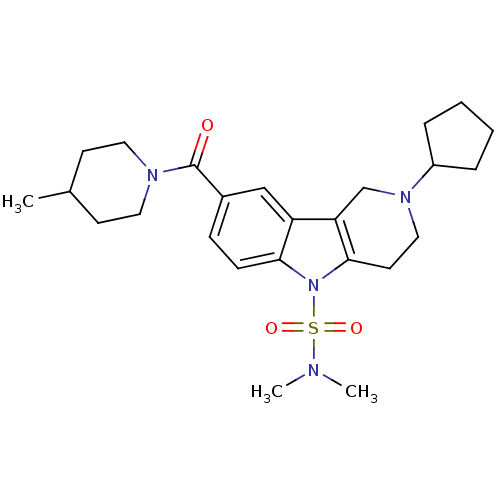
(CHEMBL1950354)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCCC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H36N4O3S/c1-18-10-13-27(14-11-18)25(30)19-8-9-23-21(16-19)22-17-28(20-6-4-5-7-20)15-12-24(22)29(23)33(31,32)26(2)3/h8-9,16,18,20H,4-7,10-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364937

(CHEMBL1950353)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H35N3O4S/c1-21-9-14-30(15-10-21)29(33)22-7-8-27-25(19-22)26-20-31(23-12-17-36-18-13-23)16-11-28(26)32(27)37(34,35)24-5-3-2-4-6-24/h2-8,19,21,23H,9-18,20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364928
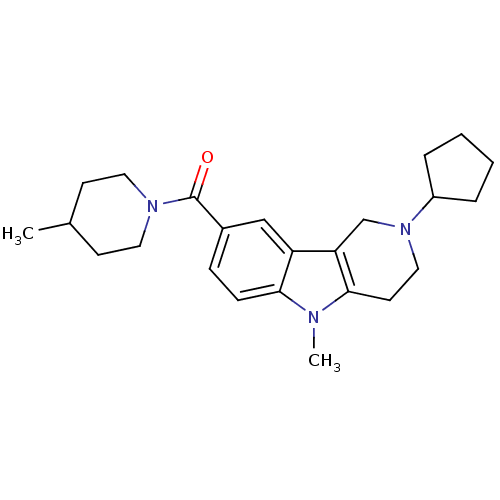
(CHEMBL1950343)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C)c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C24H33N3O/c1-17-9-12-26(13-10-17)24(28)18-7-8-22-20(15-18)21-16-27(19-5-3-4-6-19)14-11-23(21)25(22)2/h7-8,15,17,19H,3-6,9-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364929

(CHEMBL1950344)Show SMILES CCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C25H35N3O/c1-3-28-23-9-8-19(25(29)26-13-10-18(2)11-14-26)16-21(23)22-17-27(15-12-24(22)28)20-6-4-5-7-20/h8-9,16,18,20H,3-7,10-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50218116

(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364933

(CHEMBL1950348)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCCC1)S(C)(=O)=O Show InChI InChI=1S/C24H33N3O3S/c1-17-9-12-25(13-10-17)24(28)18-7-8-22-20(15-18)21-16-26(19-5-3-4-6-19)14-11-23(21)27(22)31(2,29)30/h7-8,15,17,19H,3-6,9-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364941
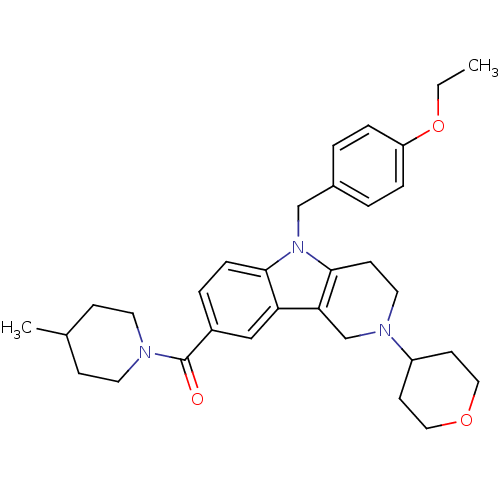
(CHEMBL1950357)Show SMILES CCOc1ccc(Cn2c3CCN(Cc3c3cc(ccc23)C(=O)N2CCC(C)CC2)C2CCOCC2)cc1 Show InChI InChI=1S/C32H41N3O3/c1-3-38-27-7-4-24(5-8-27)21-35-30-9-6-25(32(36)33-15-10-23(2)11-16-33)20-28(30)29-22-34(17-12-31(29)35)26-13-18-37-19-14-26/h4-9,20,23,26H,3,10-19,21-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364924

(CHEMBL1950335)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCCCC1 Show InChI InChI=1S/C27H37N3O/c1-3-14-30-25-10-9-21(27(31)28-15-11-20(2)12-16-28)18-23(25)24-19-29(17-13-26(24)30)22-7-5-4-6-8-22/h3,9-10,18,20,22H,1,4-8,11-17,19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364926

(CHEMBL1950341)Show SMILES CCCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O2/c1-3-11-29-24-5-4-20(26(30)27-12-6-19(2)7-13-27)17-22(24)23-18-28(14-8-25(23)29)21-9-15-31-16-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364946

(CHEMBL1950489)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C)c3CCN(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C24H33N3O2/c1-17-5-10-26(11-6-17)24(28)18-3-4-22-20(15-18)21-16-27(12-7-23(21)25(22)2)19-8-13-29-14-9-19/h3-4,15,17,19H,5-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364930

(CHEMBL1950345)Show SMILES CCCC(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C27H37N3O3/c1-3-4-26(31)30-24-6-5-20(27(32)28-12-7-19(2)8-13-28)17-22(24)23-18-29(14-9-25(23)30)21-10-15-33-16-11-21/h5-6,17,19,21H,3-4,7-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364939

(CHEMBL1950355)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H36N4O4S/c1-18-6-11-27(12-7-18)25(30)19-4-5-23-21(16-19)22-17-28(20-9-14-33-15-10-20)13-8-24(22)29(23)34(31,32)26(2)3/h4-5,16,18,20H,6-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364931

(CHEMBL1950346)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O4S/c1-3-16-34(31,32)29-24-5-4-20(26(30)27-11-6-19(2)7-12-27)17-22(24)23-18-28(13-8-25(23)29)21-9-14-33-15-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364922

(CHEMBL1950330)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(CC4CC4)Cc3c2c1 Show InChI InChI=1S/C25H33N3O/c1-3-11-28-23-7-6-20(25(29)27-13-8-18(2)9-14-27)15-21(23)22-17-26(12-10-24(22)28)16-19-4-5-19/h3,6-7,15,18-19H,1,4-5,8-14,16-17H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364936

(CHEMBL1950352)Show SMILES CC(C)S(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O4S/c1-18(2)34(31,32)29-24-5-4-20(26(30)27-11-6-19(3)7-12-27)16-22(24)23-17-28(13-8-25(23)29)21-9-14-33-15-10-21/h4-5,16,18-19,21H,6-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364945
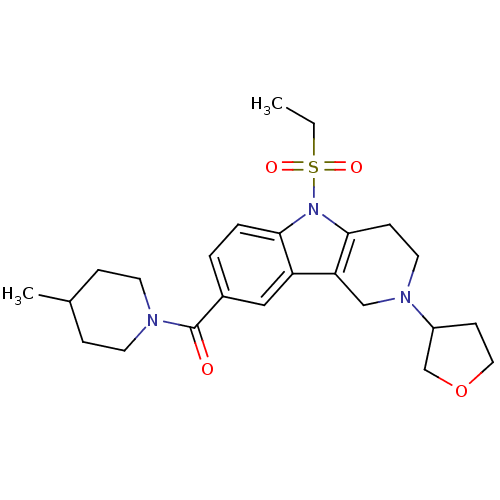
(CHEMBL1950488)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOC1 Show InChI InChI=1S/C24H33N3O4S/c1-3-32(29,30)27-22-5-4-18(24(28)25-10-6-17(2)7-11-25)14-20(22)21-15-26(12-8-23(21)27)19-9-13-31-16-19/h4-5,14,17,19H,3,6-13,15-16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364947

(CHEMBL1950490)Show SMILES Cn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCCCC1)C1CCOCC1 Show InChI InChI=1S/C23H31N3O2/c1-24-21-6-5-17(23(27)25-10-3-2-4-11-25)15-19(21)20-16-26(12-7-22(20)24)18-8-13-28-14-9-18/h5-6,15,18H,2-4,7-14,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364934
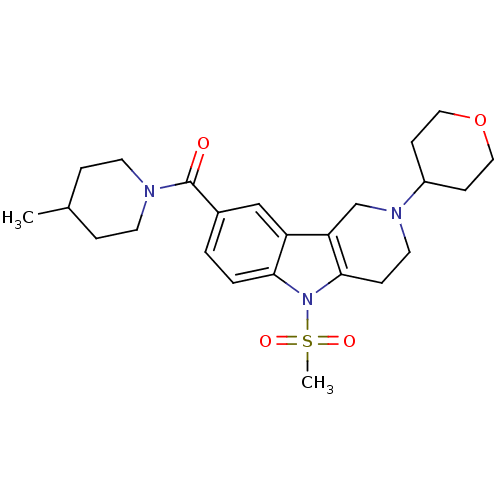
(CHEMBL1950349)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C24H33N3O4S/c1-17-5-10-25(11-6-17)24(28)18-3-4-22-20(15-18)21-16-26(19-8-13-31-14-9-19)12-7-23(21)27(22)32(2,29)30/h3-4,15,17,19H,5-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364927

(CHEMBL1950342)Show SMILES CC1CCN(CC1)C(=O)c1ccc2[nH]c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C23H31N3O/c1-16-8-11-25(12-9-16)23(27)17-6-7-21-19(14-17)20-15-26(13-10-22(20)24-21)18-4-2-3-5-18/h6-7,14,16,18,24H,2-5,8-13,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364944

(CHEMBL1950487)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-14-33(30,31)28-23-5-4-19(25(29)26-10-6-18(2)7-11-26)15-21(23)22-16-27(12-8-24(22)28)20-9-13-32-17-20/h4-5,15,18,20H,3,6-14,16-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364942

(CHEMBL1950485)Show SMILES CCNS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H36N4O4S/c1-3-26-34(31,32)29-23-5-4-19(25(30)27-11-6-18(2)7-12-27)16-21(23)22-17-28(13-8-24(22)29)20-9-14-33-15-10-20/h4-5,16,18,20,26H,3,6-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364943

(CHEMBL1950486)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)n1ccnc1 Show InChI InChI=1S/C26H33N5O4S/c1-19-4-10-28(11-5-19)26(32)20-2-3-24-22(16-20)23-17-29(21-7-14-35-15-8-21)12-6-25(23)31(24)36(33,34)30-13-9-27-18-30/h2-3,9,13,16,18-19,21H,4-8,10-12,14-15,17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364940
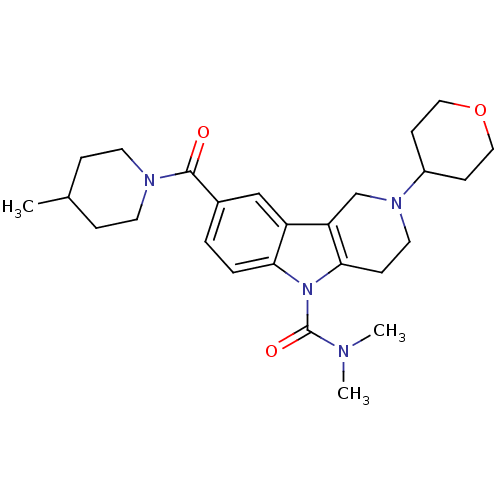
(CHEMBL1950356)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C(=O)N(C)C)c3CCN(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C26H36N4O3/c1-18-6-11-28(12-7-18)25(31)19-4-5-23-21(16-19)22-17-29(20-9-14-33-15-10-20)13-8-24(22)30(23)26(32)27(2)3/h4-5,16,18,20H,6-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor in field-stimulated mouse vas deferens |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364931

(CHEMBL1950346)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O4S/c1-3-16-34(31,32)29-24-5-4-20(26(30)27-11-6-19(2)7-12-27)17-22(24)23-18-28(13-8-25(23)29)21-9-14-33-15-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human Erg by voltage ion flux electrophysiological assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364946

(CHEMBL1950489)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C)c3CCN(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C24H33N3O2/c1-17-5-10-26(11-6-17)24(28)18-3-4-22-20(15-18)21-16-27(12-7-23(21)25(22)2)19-8-13-29-14-9-19/h3-4,15,17,19H,5-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human Erg by voltage ion flux electrophysiological assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human Erg by voltage ion flux electrophysiological assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human KOR |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human DOR |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human MOR |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364944

(CHEMBL1950487)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-14-33(30,31)28-23-5-4-19(25(29)26-10-6-18(2)7-11-26)15-21(23)22-16-27(12-8-24(22)28)20-9-13-32-17-20/h4-5,15,18,20H,3,6-14,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human Erg by voltage ion flux electrophysiological assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364947

(CHEMBL1950490)Show SMILES Cn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCCCC1)C1CCOCC1 Show InChI InChI=1S/C23H31N3O2/c1-24-21-6-5-17(23(27)25-10-3-2-4-11-25)15-19(21)20-16-26(12-7-22(20)24)18-8-13-28-14-9-18/h5-6,15,18H,2-4,7-14,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human Erg by voltage ion flux electrophysiological assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364945

(CHEMBL1950488)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOC1 Show InChI InChI=1S/C24H33N3O4S/c1-3-32(29,30)27-22-5-4-18(24(28)25-10-6-17(2)7-11-25)14-20(22)21-15-26(12-8-23(21)27)19-9-13-31-16-19/h4-5,14,17,19H,3,6-13,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Inhibition of human Erg by voltage ion flux electrophysiological assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364924

(CHEMBL1950335)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCCCC1 Show InChI InChI=1S/C27H37N3O/c1-3-14-30-25-10-9-21(27(31)28-15-11-20(2)12-16-28)18-23(25)24-19-29(17-13-26(24)30)22-7-5-4-6-8-22/h3,9-10,18,20,22H,1,4-8,11-17,19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364928

(CHEMBL1950343)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C)c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C24H33N3O/c1-17-9-12-26(13-10-17)24(28)18-7-8-22-20(15-18)21-16-27(19-5-3-4-6-19)14-11-23(21)25(22)2/h7-8,15,17,19H,3-6,9-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364932

(CHEMBL1950347)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C26H37N3O3S/c1-3-16-33(31,32)29-24-9-8-20(26(30)27-13-10-19(2)11-14-27)17-22(24)23-18-28(15-12-25(23)29)21-6-4-5-7-21/h8-9,17,19,21H,3-7,10-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364938

(CHEMBL1950354)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCCC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H36N4O3S/c1-18-10-13-27(14-11-18)25(30)19-8-9-23-21(16-19)22-17-28(20-6-4-5-7-20)15-12-24(22)29(23)33(31,32)26(2)3/h8-9,16,18,20H,4-7,10-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364934

(CHEMBL1950349)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C24H33N3O4S/c1-17-5-10-25(11-6-17)24(28)18-3-4-22-20(15-18)21-16-26(19-8-13-31-14-9-19)12-7-23(21)27(22)32(2,29)30/h3-4,15,17,19H,5-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 473 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364951

(CHEMBL1950336)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O2/c1-3-11-29-24-5-4-20(26(30)27-12-6-19(2)7-13-27)17-22(24)23-18-28(14-8-25(23)29)21-9-15-31-16-10-21/h3-5,17,19,21H,1,6-16,18H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50364922

(CHEMBL1950330)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(CC4CC4)Cc3c2c1 Show InChI InChI=1S/C25H33N3O/c1-3-11-28-23-7-6-20(25(29)27-13-8-18(2)9-14-27)15-21(23)22-17-26(12-10-24(22)28)16-19-4-5-19/h3,6-7,15,18-19H,1,4-5,8-14,16-17H2,2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor in rat brain tissue |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364937

(CHEMBL1950353)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H35N3O4S/c1-21-9-14-30(15-10-21)29(33)22-7-8-27-25(19-22)26-20-31(23-12-17-36-18-13-23)16-11-28(26)32(27)37(34,35)24-5-3-2-4-6-24/h2-8,19,21,23H,9-18,20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364936

(CHEMBL1950352)Show SMILES CC(C)S(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O4S/c1-18(2)34(31,32)29-24-5-4-20(26(30)27-11-6-19(3)7-12-27)16-22(24)23-17-28(13-8-25(23)29)21-9-14-33-15-10-21/h4-5,16,18-19,21H,6-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364926

(CHEMBL1950341)Show SMILES CCCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O2/c1-3-11-29-24-5-4-20(26(30)27-12-6-19(2)7-13-27)17-22(24)23-18-28(14-8-25(23)29)21-9-15-31-16-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364949
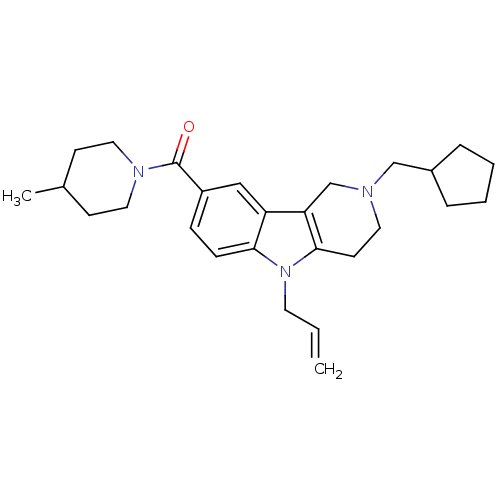
(CHEMBL1950332)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(CC4CCCC4)Cc3c2c1 Show InChI InChI=1S/C27H37N3O/c1-3-13-30-25-9-8-22(27(31)29-15-10-20(2)11-16-29)17-23(25)24-19-28(14-12-26(24)30)18-21-6-4-5-7-21/h3,8-9,17,20-21H,1,4-7,10-16,18-19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364922

(CHEMBL1950330)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(CC4CC4)Cc3c2c1 Show InChI InChI=1S/C25H33N3O/c1-3-11-28-23-7-6-20(25(29)27-13-8-18(2)9-14-27)15-21(23)22-17-26(12-10-24(22)28)16-19-4-5-19/h3,6-7,15,18-19H,1,4-5,8-14,16-17H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 768 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364941

(CHEMBL1950357)Show SMILES CCOc1ccc(Cn2c3CCN(Cc3c3cc(ccc23)C(=O)N2CCC(C)CC2)C2CCOCC2)cc1 Show InChI InChI=1S/C32H41N3O3/c1-3-38-27-7-4-24(5-8-27)21-35-30-9-6-25(32(36)33-15-10-23(2)11-16-33)20-28(30)29-22-34(17-12-31(29)35)26-13-18-37-19-14-26/h4-9,20,23,26H,3,10-19,21-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364953

(CHEMBL1950338)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc4ccccn4)Cc3c2c1 Show InChI InChI=1S/C27H32N4O/c1-3-13-31-25-8-7-21(27(32)30-15-9-20(2)10-16-30)17-23(25)24-19-29(14-11-26(24)31)18-22-6-4-5-12-28-22/h3-8,12,17,20H,1,9-11,13-16,18-19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.42E+3 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364929

(CHEMBL1950344)Show SMILES CCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C25H35N3O/c1-3-28-23-9-8-19(25(29)26-13-10-18(2)11-14-26)16-21(23)22-17-27(15-12-24(22)28)20-6-4-5-7-20/h8-9,16,18,20H,3-7,10-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364948
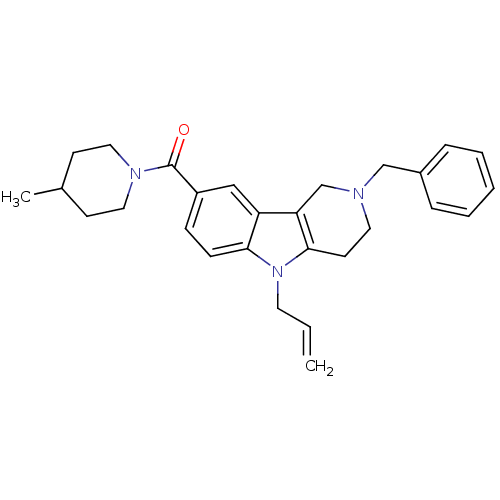
(CHEMBL1950331)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc4ccccc4)Cc3c2c1 Show InChI InChI=1S/C28H33N3O/c1-3-14-31-26-10-9-23(28(32)30-16-11-21(2)12-17-30)18-24(26)25-20-29(15-13-27(25)31)19-22-7-5-4-6-8-22/h3-10,18,21H,1,11-17,19-20H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364933

(CHEMBL1950348)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCCC1)S(C)(=O)=O Show InChI InChI=1S/C24H33N3O3S/c1-17-9-12-25(13-10-17)24(28)18-7-8-22-20(15-18)21-16-26(19-5-3-4-6-19)14-11-23(21)27(22)31(2,29)30/h7-8,15,17,19H,3-6,9-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364923

(CHEMBL1950333)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C26H35N3O/c1-3-13-29-24-9-8-20(26(30)27-14-10-19(2)11-15-27)17-22(24)23-18-28(16-12-25(23)29)21-6-4-5-7-21/h3,8-9,17,19,21H,1,4-7,10-16,18H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364939

(CHEMBL1950355)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H36N4O4S/c1-18-6-11-27(12-7-18)25(30)19-4-5-23-21(16-19)22-17-28(20-9-14-33-15-10-20)13-8-24(22)29(23)34(31,32)26(2)3/h4-5,16,18,20H,6-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364927

(CHEMBL1950342)Show SMILES CC1CCN(CC1)C(=O)c1ccc2[nH]c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C23H31N3O/c1-16-8-11-25(12-9-16)23(27)17-6-7-21-19(14-17)20-15-26(13-10-22(20)24-21)18-4-2-3-5-18/h6-7,14,16,18,24H,2-5,8-13,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.25E+3 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50218116

(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364930

(CHEMBL1950345)Show SMILES CCCC(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C27H37N3O3/c1-3-4-26(31)30-24-6-5-20(27(32)28-12-7-19(2)8-13-28)17-22(24)23-18-29(14-9-25(23)30)21-10-15-33-16-11-21/h5-6,17,19,21H,3-4,7-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Partial agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364925

(CHEMBL1950340)Show SMILES CCCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C26H37N3O/c1-3-13-29-24-9-8-20(26(30)27-14-10-19(2)11-15-27)17-22(24)23-18-28(16-12-25(23)29)21-6-4-5-7-21/h8-9,17,19,21H,3-7,10-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364954
(CHEMBL1950339)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc4ccncc4)Cc3c2c1 Show InChI InChI=1S/C27H32N4O/c1-3-13-31-25-5-4-22(27(32)30-15-8-20(2)9-16-30)17-23(25)24-19-29(14-10-26(24)31)18-21-6-11-28-12-7-21/h3-7,11-12,17,20H,1,8-10,13-16,18-19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364952
(CHEMBL1950337)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc4cccnc4)Cc3c2c1 Show InChI InChI=1S/C27H32N4O/c1-3-12-31-25-7-6-22(27(32)30-14-8-20(2)9-15-30)16-23(25)24-19-29(13-10-26(24)31)18-21-5-4-11-28-17-21/h3-7,11,16-17,20H,1,8-10,12-15,18-19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364950
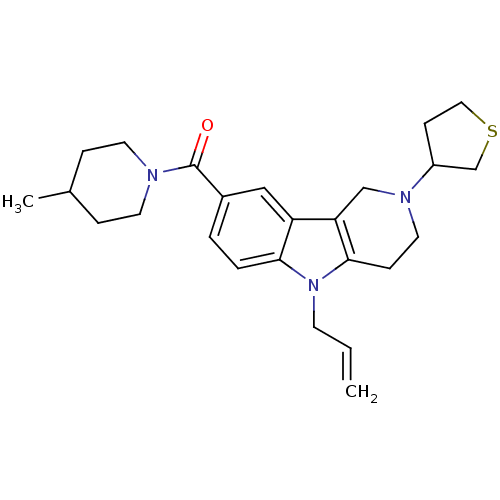
(CHEMBL1950334)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCSC1 Show InChI InChI=1S/C25H33N3OS/c1-3-10-28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)15-21(23)22-16-27(13-8-24(22)28)20-9-14-30-17-20/h3-5,15,18,20H,1,6-14,16-17H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor in rat brain tissue |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364935

(CHEMBL1950350)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C25H35N3O3S/c1-3-32(30,31)28-23-9-8-19(25(29)26-13-10-18(2)11-14-26)16-21(23)22-17-27(15-12-24(22)28)20-6-4-5-7-20/h8-9,16,18,20H,3-7,10-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364931

(CHEMBL1950346)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O4S/c1-3-16-34(31,32)29-24-5-4-20(26(30)27-11-6-19(2)7-12-27)17-22(24)23-18-28(13-8-25(23)29)21-9-14-33-15-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 129 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364921

(CHEMBL1950329)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCCC2)N(C)S(=O)(=O)c2cccs2)nc1 Show InChI InChI=1S/C27H32N4O3S2/c1-3-34-23-13-11-21(28-18-23)16-26-29-24-17-22(30(2)36(32,33)27-10-7-15-35-27)12-14-25(24)31(26)19-20-8-5-4-6-9-20/h7,10-15,17-18,20H,3-6,8-9,16,19H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assay |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data