 Found 96 hits of Enzyme Inhibition Constant Data
Found 96 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053590
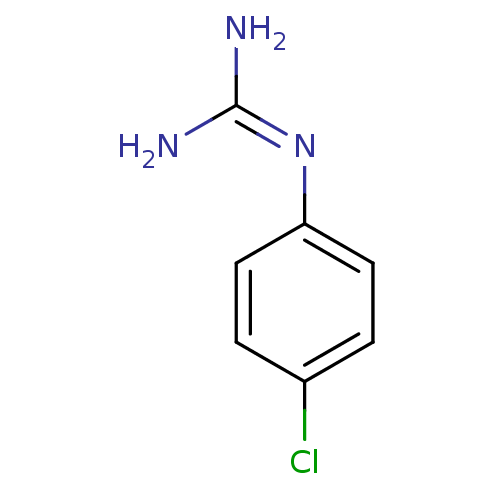
(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053590

(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16128
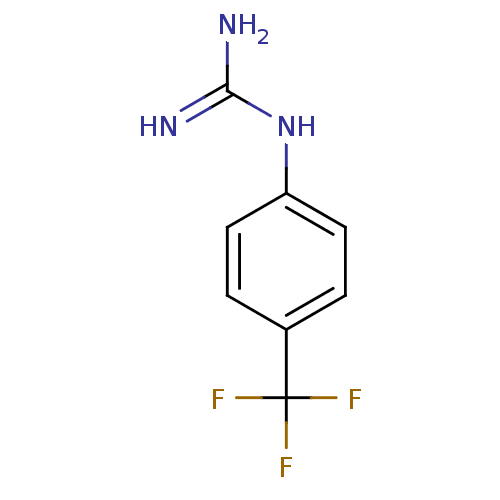
(4-(trifluoromethyl)benzene-1-guanidine | CHEMBL439...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-1-3-6(4-2-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16128

(4-(trifluoromethyl)benzene-1-guanidine | CHEMBL439...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-1-3-6(4-2-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50100962
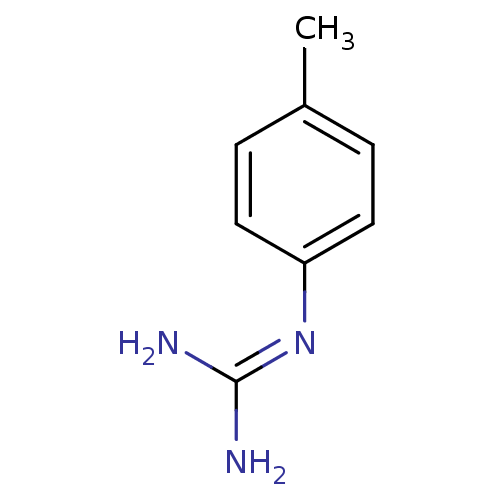
(1N-amino(immino)methyl-4-methylaniline | CHEMBL434...)Show InChI InChI=1S/C8H11N3/c1-6-2-4-7(5-3-6)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50100962

(1N-amino(immino)methyl-4-methylaniline | CHEMBL434...)Show InChI InChI=1S/C8H11N3/c1-6-2-4-7(5-3-6)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014339
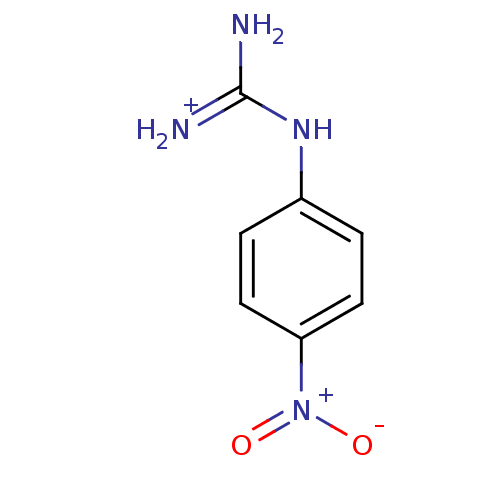
(1N-amino(immino)methyl-4-nitroaniline)Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-1-3-6(4-2-5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053620

(1-phenylguanidine | 1N-amino(immino)methylaniline ...)Show InChI InChI=1S/C7H9N3/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H4,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053620

(1-phenylguanidine | 1N-amino(immino)methylaniline ...)Show InChI InChI=1S/C7H9N3/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H4,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014339

(1N-amino(immino)methyl-4-nitroaniline)Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-1-3-6(4-2-5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014343
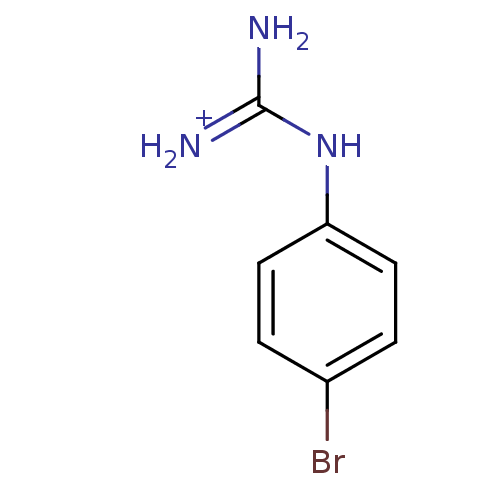
(1N-amino(immino)methyl-4-bromoaniline)Show InChI InChI=1S/C7H8BrN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014343

(1N-amino(immino)methyl-4-bromoaniline)Show InChI InChI=1S/C7H8BrN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014337

(1N-amino(immino)methyl-2-nitroaniline)Show SMILES [#7]\[#6](-[#7])=[#7+]/c1ccccc1-[#7+](-[#8-])=O Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-3-1-2-4-6(5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014337

(1N-amino(immino)methyl-2-nitroaniline)Show SMILES [#7]\[#6](-[#7])=[#7+]/c1ccccc1-[#7+](-[#8-])=O Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-3-1-2-4-6(5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053608
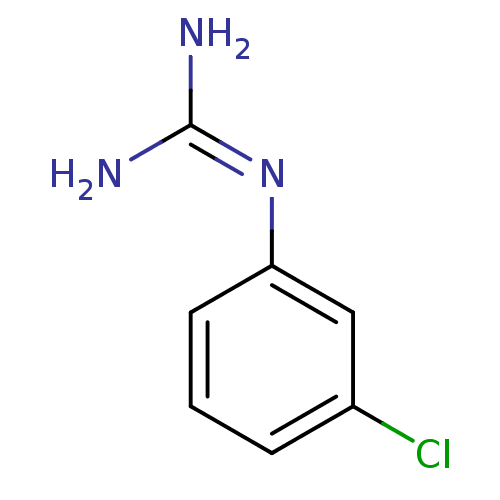
(1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...)Show InChI InChI=1S/C7H8ClN3/c8-5-2-1-3-6(4-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053608

(1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...)Show InChI InChI=1S/C7H8ClN3/c8-5-2-1-3-6(4-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM16128

(4-(trifluoromethyl)benzene-1-guanidine | CHEMBL439...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-1-3-6(4-2-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM16128

(4-(trifluoromethyl)benzene-1-guanidine | CHEMBL439...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-1-3-6(4-2-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014344
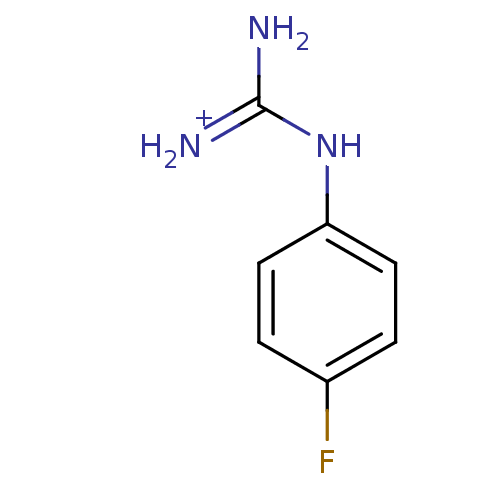
(1N-amino(immino)methyl-4-fluoroaniline)Show InChI InChI=1S/C7H8FN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 7.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014344

(1N-amino(immino)methyl-4-fluoroaniline)Show InChI InChI=1S/C7H8FN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 7.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014326
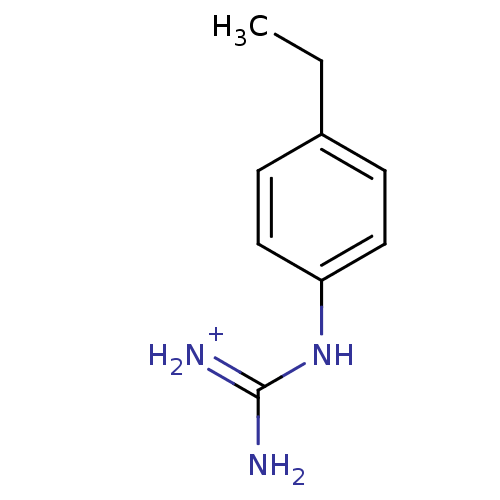
(1N-amino(immino)methyl-4-ethylaniline)Show InChI InChI=1S/C9H13N3/c1-2-7-3-5-8(6-4-7)12-9(10)11/h3-6H,2H2,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014326

(1N-amino(immino)methyl-4-ethylaniline)Show InChI InChI=1S/C9H13N3/c1-2-7-3-5-8(6-4-7)12-9(10)11/h3-6H,2H2,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053633
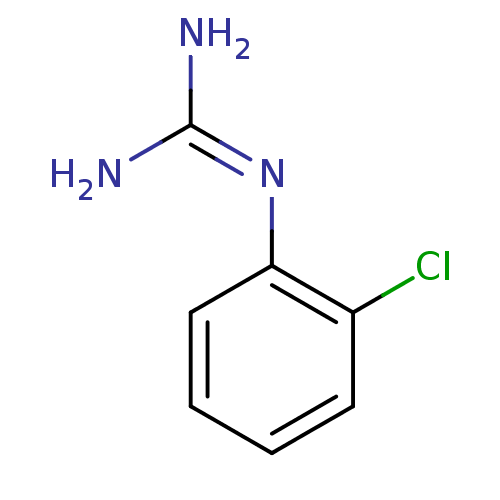
(1N-amino(immino)methyl-2-chloroaniline | CHEMBL418...)Show InChI InChI=1S/C7H8ClN3/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053633

(1N-amino(immino)methyl-2-chloroaniline | CHEMBL418...)Show InChI InChI=1S/C7H8ClN3/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053590

(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053590

(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014337

(1N-amino(immino)methyl-2-nitroaniline)Show SMILES [#7]\[#6](-[#7])=[#7+]/c1ccccc1-[#7+](-[#8-])=O Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-3-1-2-4-6(5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014337

(1N-amino(immino)methyl-2-nitroaniline)Show SMILES [#7]\[#6](-[#7])=[#7+]/c1ccccc1-[#7+](-[#8-])=O Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-3-1-2-4-6(5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014340
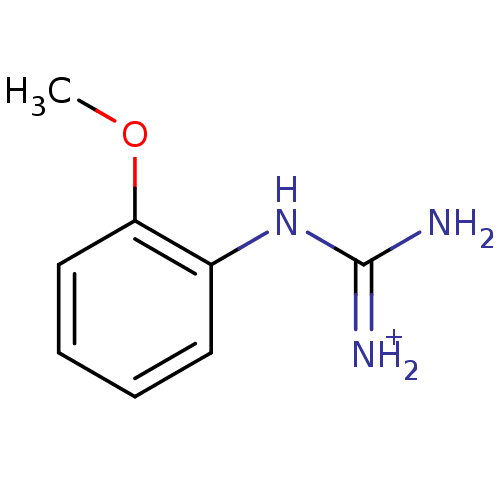
(1N-amino(immino)methyl-2-methoxyaniline)Show InChI InChI=1S/C8H11N3O/c1-12-7-5-3-2-4-6(7)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014340

(1N-amino(immino)methyl-2-methoxyaniline)Show InChI InChI=1S/C8H11N3O/c1-12-7-5-3-2-4-6(7)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053620

(1-phenylguanidine | 1N-amino(immino)methylaniline ...)Show InChI InChI=1S/C7H9N3/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H4,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053620

(1-phenylguanidine | 1N-amino(immino)methylaniline ...)Show InChI InChI=1S/C7H9N3/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H4,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014332

(1N-amino(immino)methyl-2-methylaniline)Show InChI InChI=1S/C8H11N3/c1-6-4-2-3-5-7(6)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014332

(1N-amino(immino)methyl-2-methylaniline)Show InChI InChI=1S/C8H11N3/c1-6-4-2-3-5-7(6)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50100962

(1N-amino(immino)methyl-4-methylaniline | CHEMBL434...)Show InChI InChI=1S/C8H11N3/c1-6-2-4-7(5-3-6)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50100962

(1N-amino(immino)methyl-4-methylaniline | CHEMBL434...)Show InChI InChI=1S/C8H11N3/c1-6-2-4-7(5-3-6)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014339

(1N-amino(immino)methyl-4-nitroaniline)Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-1-3-6(4-2-5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 3.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014339

(1N-amino(immino)methyl-4-nitroaniline)Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-1-3-6(4-2-5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 3.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014329
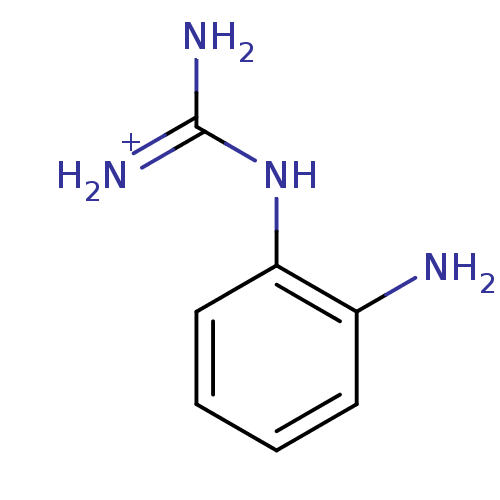
(1N-amino(immino)methyl-1,2-benzenediamine)Show InChI InChI=1S/C7H10N4/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,8H2,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014329

(1N-amino(immino)methyl-1,2-benzenediamine)Show InChI InChI=1S/C7H10N4/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,8H2,(H4,9,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053603
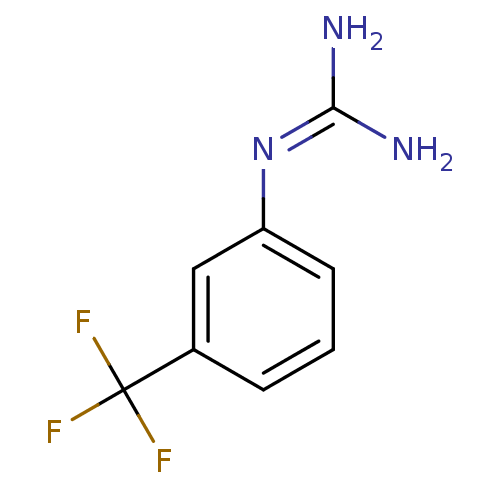
(1N-amino(immino)methyl-3-trifluoromethylaniline | ...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-2-1-3-6(4-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053603

(1N-amino(immino)methyl-3-trifluoromethylaniline | ...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-2-1-3-6(4-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014330
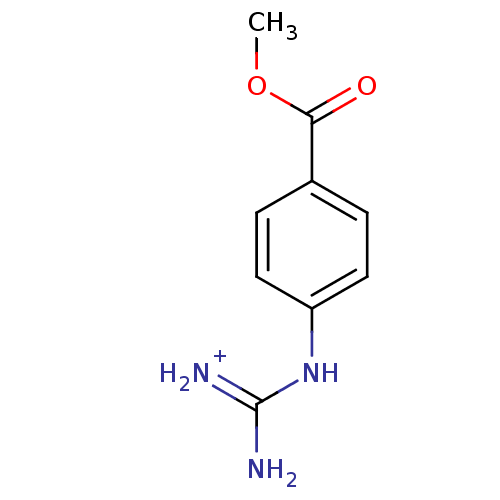
(methyl 4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C9H11N3O2/c1-14-8(13)6-2-4-7(5-3-6)12-9(10)11/h2-5H,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014330

(methyl 4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C9H11N3O2/c1-14-8(13)6-2-4-7(5-3-6)12-9(10)11/h2-5H,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50100969
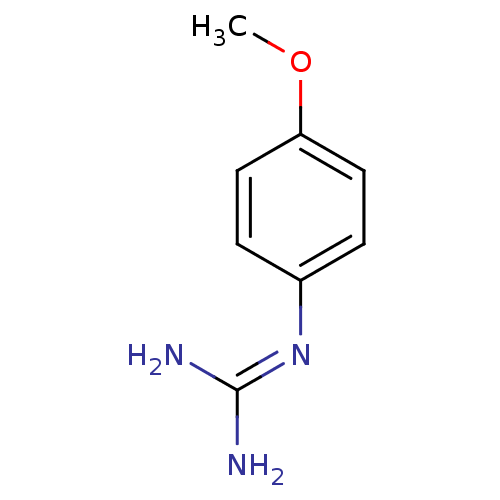
(1N-amino(immino)methyl-4-methoxyaniline | CHEMBL29...)Show InChI InChI=1S/C8H11N3O/c1-12-7-4-2-6(3-5-7)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50100969

(1N-amino(immino)methyl-4-methoxyaniline | CHEMBL29...)Show InChI InChI=1S/C8H11N3O/c1-12-7-4-2-6(3-5-7)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053620

(1-phenylguanidine | 1N-amino(immino)methylaniline ...)Show InChI InChI=1S/C7H9N3/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H4,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibition against Tissue plasminogen activator at 1 mM when assayed against 0.3 mM S-2288 (Km = 0.673 mM) |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014341
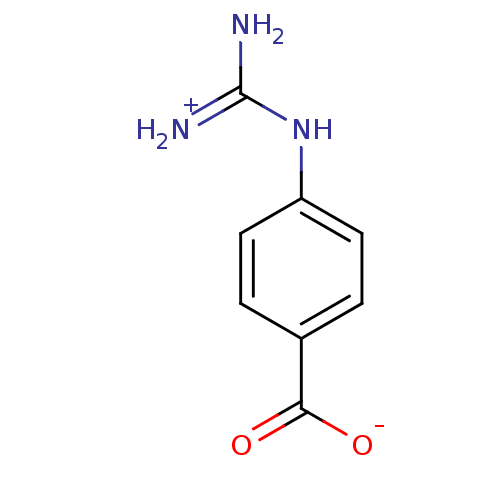
(4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C8H9N3O2/c9-8(10)11-6-3-1-5(2-4-6)7(12)13/h1-4H,(H,12,13)(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014341

(4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C8H9N3O2/c9-8(10)11-6-3-1-5(2-4-6)7(12)13/h1-4H,(H,12,13)(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014344

(1N-amino(immino)methyl-4-fluoroaniline)Show InChI InChI=1S/C7H8FN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 8.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014344

(1N-amino(immino)methyl-4-fluoroaniline)Show InChI InChI=1S/C7H8FN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 8.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014340

(1N-amino(immino)methyl-2-methoxyaniline)Show InChI InChI=1S/C8H11N3O/c1-12-7-5-3-2-4-6(7)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014340

(1N-amino(immino)methyl-2-methoxyaniline)Show InChI InChI=1S/C8H11N3O/c1-12-7-5-3-2-4-6(7)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053633

(1N-amino(immino)methyl-2-chloroaniline | CHEMBL418...)Show InChI InChI=1S/C7H8ClN3/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053633

(1N-amino(immino)methyl-2-chloroaniline | CHEMBL418...)Show InChI InChI=1S/C7H8ClN3/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053608

(1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...)Show InChI InChI=1S/C7H8ClN3/c8-5-2-1-3-6(4-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053608

(1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...)Show InChI InChI=1S/C7H8ClN3/c8-5-2-1-3-6(4-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014329

(1N-amino(immino)methyl-1,2-benzenediamine)Show InChI InChI=1S/C7H10N4/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,8H2,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014329

(1N-amino(immino)methyl-1,2-benzenediamine)Show InChI InChI=1S/C7H10N4/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,8H2,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014331
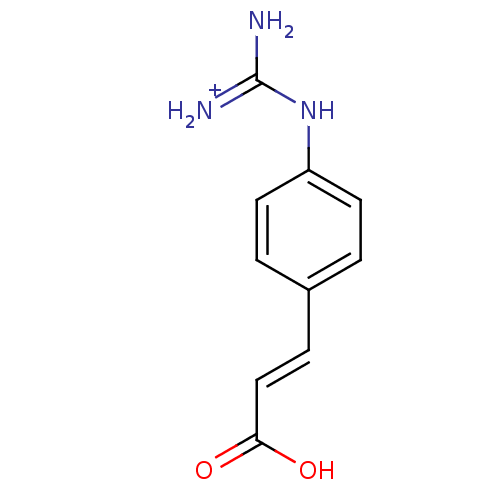
(3-[4-amino(immino)methylaminophenyl]-(E)-2-propeno...)Show InChI InChI=1S/C10H11N3O2/c11-10(12)13-8-4-1-7(2-5-8)3-6-9(14)15/h1-6H,(H,14,15)(H4,11,12,13)/p+1/b6-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 1.32E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014331

(3-[4-amino(immino)methylaminophenyl]-(E)-2-propeno...)Show InChI InChI=1S/C10H11N3O2/c11-10(12)13-8-4-1-7(2-5-8)3-6-9(14)15/h1-6H,(H,14,15)(H4,11,12,13)/p+1/b6-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 1.33E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014330

(methyl 4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C9H11N3O2/c1-14-8(13)6-2-4-7(5-3-6)12-9(10)11/h2-5H,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50014330

(methyl 4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C9H11N3O2/c1-14-8(13)6-2-4-7(5-3-6)12-9(10)11/h2-5H,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Urokinase-type plasminogen activator |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50053620

(1-phenylguanidine | 1N-amino(immino)methylaniline ...)Show InChI InChI=1S/C7H9N3/c8-7(9)10-6-4-2-1-3-5-6/h1-5H,(H4,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.61E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014343

(1N-amino(immino)methyl-4-bromoaniline)Show InChI InChI=1S/C7H8BrN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.78E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014343

(1N-amino(immino)methyl-4-bromoaniline)Show InChI InChI=1S/C7H8BrN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.78E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014332

(1N-amino(immino)methyl-2-methylaniline)Show InChI InChI=1S/C8H11N3/c1-6-4-2-3-5-7(6)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014332

(1N-amino(immino)methyl-2-methylaniline)Show InChI InChI=1S/C8H11N3/c1-6-4-2-3-5-7(6)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014331

(3-[4-amino(immino)methylaminophenyl]-(E)-2-propeno...)Show InChI InChI=1S/C10H11N3O2/c11-10(12)13-8-4-1-7(2-5-8)3-6-9(14)15/h1-6H,(H,14,15)(H4,11,12,13)/p+1/b6-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 4.92E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014331

(3-[4-amino(immino)methylaminophenyl]-(E)-2-propeno...)Show InChI InChI=1S/C10H11N3O2/c11-10(12)13-8-4-1-7(2-5-8)3-6-9(14)15/h1-6H,(H,14,15)(H4,11,12,13)/p+1/b6-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 4.92E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014326

(1N-amino(immino)methyl-4-ethylaniline)Show InChI InChI=1S/C9H13N3/c1-2-7-3-5-8(6-4-7)12-9(10)11/h3-6H,2H2,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.77E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014326

(1N-amino(immino)methyl-4-ethylaniline)Show InChI InChI=1S/C9H13N3/c1-2-7-3-5-8(6-4-7)12-9(10)11/h3-6H,2H2,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.77E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50100969

(1N-amino(immino)methyl-4-methoxyaniline | CHEMBL29...)Show InChI InChI=1S/C8H11N3O/c1-12-7-4-2-6(3-5-7)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.89E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50100969

(1N-amino(immino)methyl-4-methoxyaniline | CHEMBL29...)Show InChI InChI=1S/C8H11N3O/c1-12-7-4-2-6(3-5-7)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.89E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50100969

(1N-amino(immino)methyl-4-methoxyaniline | CHEMBL29...)Show InChI InChI=1S/C8H11N3O/c1-12-7-4-2-6(3-5-7)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50053633

(1N-amino(immino)methyl-2-chloroaniline | CHEMBL418...)Show InChI InChI=1S/C7H8ClN3/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014329

(1N-amino(immino)methyl-1,2-benzenediamine)Show InChI InChI=1S/C7H10N4/c8-5-3-1-2-4-6(5)11-7(9)10/h1-4H,8H2,(H4,9,10,11)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014341

(4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C8H9N3O2/c9-8(10)11-6-3-1-5(2-4-6)7(12)13/h1-4H,(H,12,13)(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014326

(1N-amino(immino)methyl-4-ethylaniline)Show InChI InChI=1S/C9H13N3/c1-2-7-3-5-8(6-4-7)12-9(10)11/h3-6H,2H2,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50053590

(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014331

(3-[4-amino(immino)methylaminophenyl]-(E)-2-propeno...)Show InChI InChI=1S/C10H11N3O2/c11-10(12)13-8-4-1-7(2-5-8)3-6-9(14)15/h1-6H,(H,14,15)(H4,11,12,13)/p+1/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50053603

(1N-amino(immino)methyl-3-trifluoromethylaniline | ...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-2-1-3-6(4-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50014341

(4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C8H9N3O2/c9-8(10)11-6-3-1-5(2-4-6)7(12)13/h1-4H,(H,12,13)(H4,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50100962

(1N-amino(immino)methyl-4-methylaniline | CHEMBL434...)Show InChI InChI=1S/C8H11N3/c1-6-2-4-7(5-3-6)11-8(9)10/h2-5H,1H3,(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50053608

(1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...)Show InChI InChI=1S/C7H8ClN3/c8-5-2-1-3-6(4-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014341

(4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C8H9N3O2/c9-8(10)11-6-3-1-5(2-4-6)7(12)13/h1-4H,(H,12,13)(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM16128

(4-(trifluoromethyl)benzene-1-guanidine | CHEMBL439...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-1-3-6(4-2-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014340

(1N-amino(immino)methyl-2-methoxyaniline)Show InChI InChI=1S/C8H11N3O/c1-12-7-5-3-2-4-6(7)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014330

(methyl 4-amino(immino)methylaminobenzoate)Show InChI InChI=1S/C9H11N3O2/c1-14-8(13)6-2-4-7(5-3-6)12-9(10)11/h2-5H,1H3,(H4,10,11,12)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014332

(1N-amino(immino)methyl-2-methylaniline)Show InChI InChI=1S/C8H11N3/c1-6-4-2-3-5-7(6)11-8(9)10/h2-5H,1H3,(H4,9,10,11)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014337

(1N-amino(immino)methyl-2-nitroaniline)Show SMILES [#7]\[#6](-[#7])=[#7+]/c1ccccc1-[#7+](-[#8-])=O Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-3-1-2-4-6(5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014344

(1N-amino(immino)methyl-4-fluoroaniline)Show InChI InChI=1S/C7H8FN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014343

(1N-amino(immino)methyl-4-bromoaniline)Show InChI InChI=1S/C7H8BrN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50014339

(1N-amino(immino)methyl-4-nitroaniline)Show InChI InChI=1S/C7H8N4O2/c8-7(9)10-5-1-3-6(4-2-5)11(12)13/h1-4H,(H4,8,9,10)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human plasmin was determined at 0.5 mM |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053603

(1N-amino(immino)methyl-3-trifluoromethylaniline | ...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-2-1-3-6(4-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.07E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
log1/Ki value was calculated against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50053603

(1N-amino(immino)methyl-3-trifluoromethylaniline | ...)Show InChI InChI=1S/C8H8F3N3/c9-8(10,11)5-2-1-3-6(4-5)14-7(12)13/h1-4H,(H4,12,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.07E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Trypsin |
J Med Chem 33: 2956-61 (1990)
BindingDB Entry DOI: 10.7270/Q2GB24NB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data