 Found 39 hits of Enzyme Inhibition Constant Data
Found 39 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4566
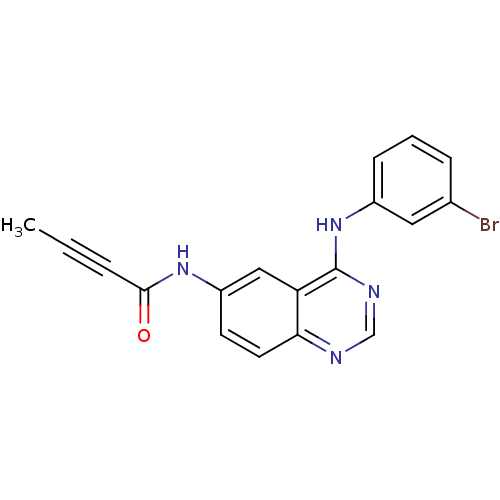
(4-anilinoquinazoline deriv. 1 | CHEMBL91867 | N-{4...)Show InChI InChI=1S/C18H13BrN4O/c1-2-4-17(24)22-14-7-8-16-15(10-14)18(21-11-20-16)23-13-6-3-5-12(19)9-13/h3,5-11H,1H3,(H,22,24)(H,20,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Btk after 60 mins |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Irreversible inhibition of EGFR |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86633
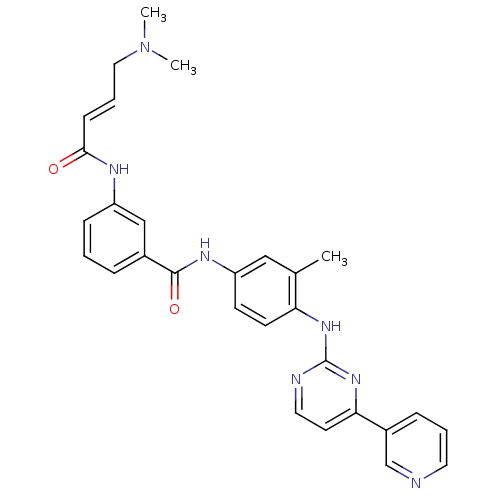
(JNK-IN-8)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)c(C)c1 Show InChI InChI=1S/C29H29N7O2/c1-20-17-24(11-12-25(20)34-29-31-15-13-26(35-29)22-8-5-14-30-19-22)33-28(38)21-7-4-9-23(18-21)32-27(37)10-6-16-36(2)3/h4-15,17-19H,16H2,1-3H3,(H,32,37)(H,33,38)(H,31,34,35)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50403056
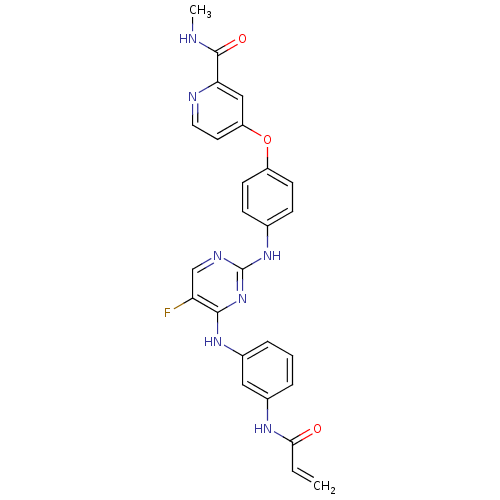
(CHEMBL2216827 | US10596172, Compound I-342 | US108...)Show SMILES CNC(=O)c1cc(Oc2ccc(Nc3ncc(F)c(Nc4cccc(NC(=O)C=C)c4)n3)cc2)ccn1 Show InChI InChI=1S/C26H22FN7O3/c1-3-23(35)31-17-5-4-6-18(13-17)32-24-21(27)15-30-26(34-24)33-16-7-9-19(10-8-16)37-20-11-12-29-22(14-20)25(36)28-2/h3-15H,1H2,2H3,(H,28,36)(H,31,35)(H2,30,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of Btk in human Ramos cells |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50403056

(CHEMBL2216827 | US10596172, Compound I-342 | US108...)Show SMILES CNC(=O)c1cc(Oc2ccc(Nc3ncc(F)c(Nc4cccc(NC(=O)C=C)c4)n3)cc2)ccn1 Show InChI InChI=1S/C26H22FN7O3/c1-3-23(35)31-17-5-4-6-18(13-17)32-24-21(27)15-30-26(34-24)33-16-7-9-19(10-8-16)37-20-11-12-29-22(14-20)25(36)28-2/h3-15H,1H2,2H3,(H,28,36)(H,31,35)(H2,30,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of Btk |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50403057
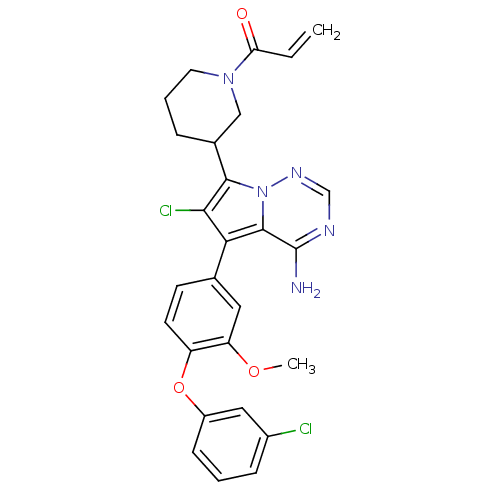
(CHEMBL2216828)Show SMILES COc1cc(ccc1Oc1cccc(Cl)c1)-c1c(Cl)c(C2CCCN(C2)C(=O)C=C)n2ncnc(N)c12 Show InChI InChI=1S/C27H25Cl2N5O3/c1-3-22(35)33-11-5-6-17(14-33)25-24(29)23(26-27(30)31-15-32-34(25)26)16-9-10-20(21(12-16)36-2)37-19-8-4-7-18(28)13-19/h3-4,7-10,12-13,15,17H,1,5-6,11,14H2,2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of Btk |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50403059

(CHEMBL2216829 | US9505766, 64)Show SMILES Cc1ccc(cc1)-c1c(\C=C(/C#N)C(=O)NC(C)(C)C)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C24H28N6O2/c1-15-6-8-16(9-7-15)19-18(12-17(13-25)23(32)29-24(2,3)4)30(10-5-11-31)22-20(19)21(26)27-14-28-22/h6-9,12,14,31H,5,10-11H2,1-4H3,(H,29,32)(H2,26,27,28)/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 transfected in HEK293 cells |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM86633

(JNK-IN-8)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)c(C)c1 Show InChI InChI=1S/C29H29N7O2/c1-20-17-24(11-12-25(20)34-29-31-15-13-26(35-29)22-8-5-14-30-19-22)33-28(38)21-7-4-9-23(18-21)32-27(37)10-6-16-36(2)3/h4-15,17-19H,16H2,1-3H3,(H,32,37)(H,33,38)(H,31,34,35)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4566

(4-anilinoquinazoline deriv. 1 | CHEMBL91867 | N-{4...)Show InChI InChI=1S/C18H13BrN4O/c1-2-4-17(24)22-14-7-8-16-15(10-14)18(21-11-20-16)23-13-6-3-5-12(19)9-13/h3,5-11H,1H3,(H,22,24)(H,20,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of EGF-induced autophosphorylation of EGFR in human A431 cells |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50403055
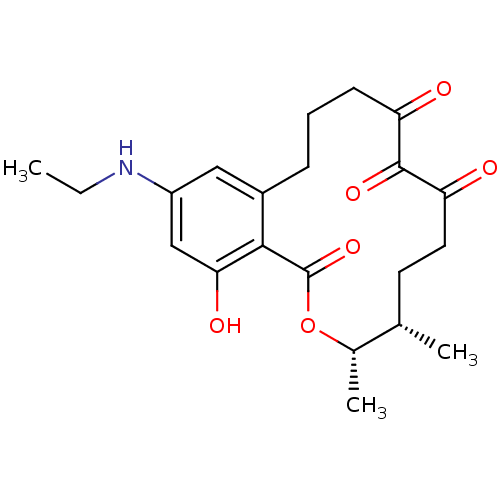
(CHEMBL2216826)Show SMILES CCNc1cc(O)c2c(CCCC(=O)C(=O)C(=O)CC[C@H](C)[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C21H27NO6/c1-4-22-15-10-14-6-5-7-16(23)20(26)17(24)9-8-12(2)13(3)28-21(27)19(14)18(25)11-15/h10-13,22,25H,4-9H2,1-3H3/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499
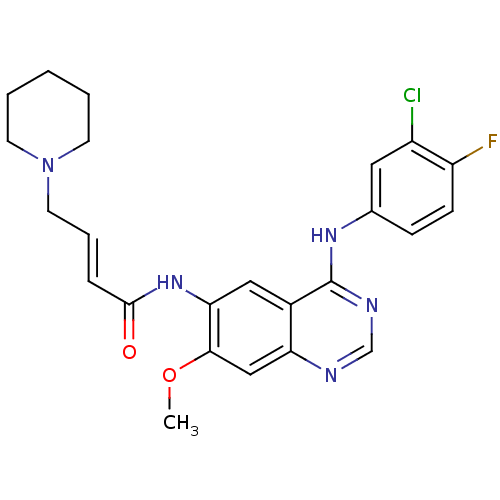
(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EGFR expressed in sf9 cells by ELISA |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50336457
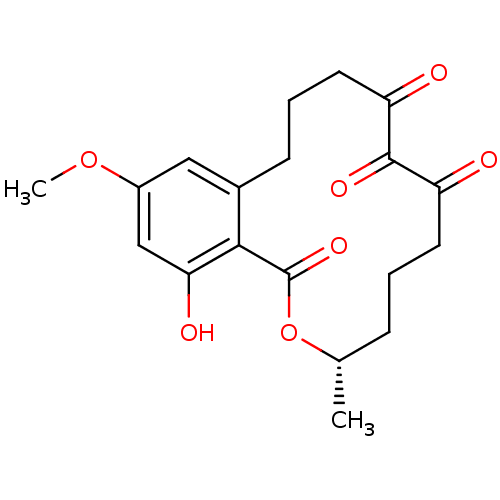
((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...)Show SMILES COc1cc(O)c2c(CCCC(=O)C(=O)C(=O)CCC[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h9-11,22H,3-8H2,1-2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50403060
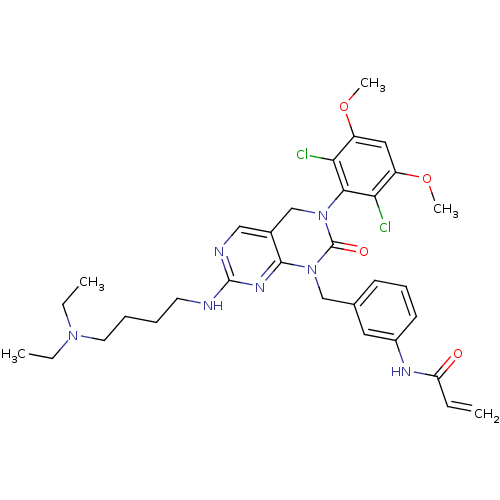
(CHEMBL2216830)Show SMILES CCN(CC)CCCCNc1ncc2CN(C(=O)N(Cc3cccc(NC(=O)C=C)c3)c2n1)c1c(Cl)c(OC)cc(OC)c1Cl Show InChI InChI=1S/C32H39Cl2N7O4/c1-6-26(42)37-23-13-11-12-21(16-23)19-41-30-22(18-36-31(38-30)35-14-9-10-15-39(7-2)8-3)20-40(32(41)43)29-27(33)24(44-4)17-25(45-5)28(29)34/h6,11-13,16-18H,1,7-10,14-15,19-20H2,2-5H3,(H,37,42)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of FGFR2 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50403060

(CHEMBL2216830)Show SMILES CCN(CC)CCCCNc1ncc2CN(C(=O)N(Cc3cccc(NC(=O)C=C)c3)c2n1)c1c(Cl)c(OC)cc(OC)c1Cl Show InChI InChI=1S/C32H39Cl2N7O4/c1-6-26(42)37-23-13-11-12-21(16-23)19-41-30-22(18-36-31(38-30)35-14-9-10-15-39(7-2)8-3)20-40(32(41)43)29-27(33)24(44-4)17-25(45-5)28(29)34/h6,11-13,16-18H,1,7-10,14-15,19-20H2,2-5H3,(H,37,42)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR1 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of ErbB4 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50403060

(CHEMBL2216830)Show SMILES CCN(CC)CCCCNc1ncc2CN(C(=O)N(Cc3cccc(NC(=O)C=C)c3)c2n1)c1c(Cl)c(OC)cc(OC)c1Cl Show InChI InChI=1S/C32H39Cl2N7O4/c1-6-26(42)37-23-13-11-12-21(16-23)19-41-30-22(18-36-31(38-30)35-14-9-10-15-39(7-2)8-3)20-40(32(41)43)29-27(33)24(44-4)17-25(45-5)28(29)34/h6,11-13,16-18H,1,7-10,14-15,19-20H2,2-5H3,(H,37,42)(H,35,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765
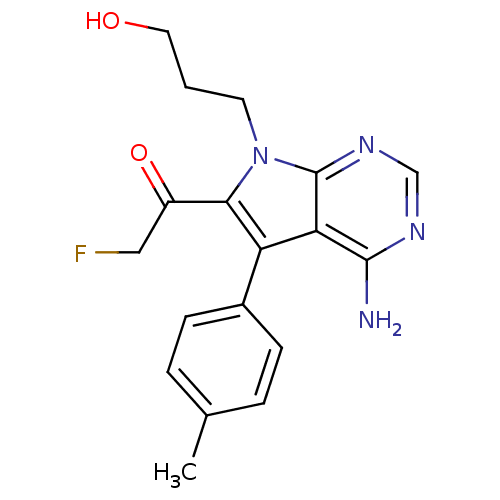
(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 transfected in HEK293 cells |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM86633

(JNK-IN-8)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)c(C)c1 Show InChI InChI=1S/C29H29N7O2/c1-20-17-24(11-12-25(20)34-29-31-15-13-26(35-29)22-8-5-14-30-19-22)33-28(38)21-7-4-9-23(18-21)32-27(37)10-6-16-36(2)3/h4-15,17-19H,16H2,1-3H3,(H,32,37)(H,33,38)(H,31,34,35)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM15234
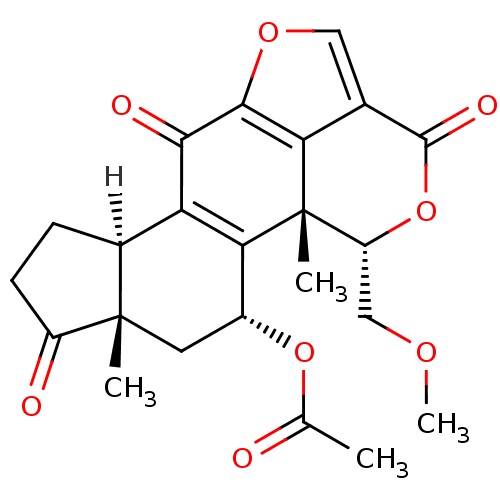
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Plk1 using recombinant protein X as a substrate containing threonine residues |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50403055

(CHEMBL2216826)Show SMILES CCNc1cc(O)c2c(CCCC(=O)C(=O)C(=O)CC[C@H](C)[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C21H27NO6/c1-4-22-15-10-14-6-5-7-16(23)20(26)17(24)9-8-12(2)13(3)28-21(27)19(14)18(25)11-15/h10-13,22,25H,4-9H2,1-3H3/t12-,13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of MEKK1 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM112499

(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged ErbB2 expressed in sf9 cells by ELISA |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM112499

(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged ErbB4 expressed in sf9 cells by ELISA |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50336457

((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...)Show SMILES COc1cc(O)c2c(CCCC(=O)C(=O)C(=O)CCC[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h9-11,22H,3-8H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of Erk2 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50403054
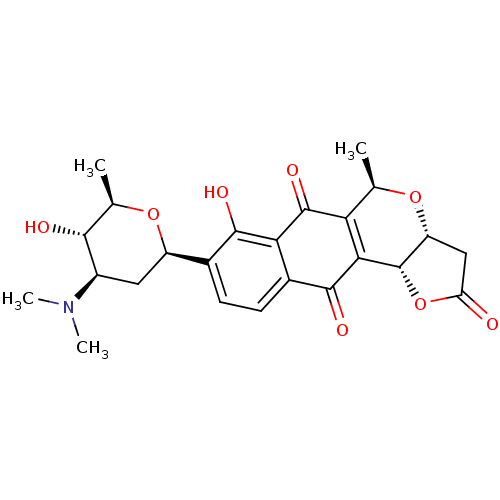
(CHEMBL2216825)Show SMILES C[C@H]1O[C@H](C[C@H]([C@@H]1O)N(C)C)c1ccc2C(=O)C3=C([C@@H](C)O[C@@H]4CC(=O)O[C@H]34)C(=O)c2c1O |r,t:18| Show InChI InChI=1S/C24H27NO8/c1-9-17-19(24-15(31-9)8-16(26)33-24)22(29)12-6-5-11(21(28)18(12)23(17)30)14-7-13(25(3)4)20(27)10(2)32-14/h5-6,9-10,13-15,20,24,27-28H,7-8H2,1-4H3/t9-,10-,13-,14-,15-,20-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 using biotinylated GSKalpha peptide (SGRARTSSFA) as a substrate after 1 hr |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50403060

(CHEMBL2216830)Show SMILES CCN(CC)CCCCNc1ncc2CN(C(=O)N(Cc3cccc(NC(=O)C=C)c3)c2n1)c1c(Cl)c(OC)cc(OC)c1Cl Show InChI InChI=1S/C32H39Cl2N7O4/c1-6-26(42)37-23-13-11-12-21(16-23)19-41-30-22(18-36-31(38-30)35-14-9-10-15-39(7-2)8-3)20-40(32(41)43)29-27(33)24(44-4)17-25(45-5)28(29)34/h6,11-13,16-18H,1,7-10,14-15,19-20H2,2-5H3,(H,37,42)(H,35,36,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of FGFR4 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 7
(Homo sapiens (Human)) | BDBM50336457

((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...)Show SMILES COc1cc(O)c2c(CCCC(=O)C(=O)C(=O)CCC[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h9-11,22H,3-8H2,1-2H3/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of MEK7 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29508
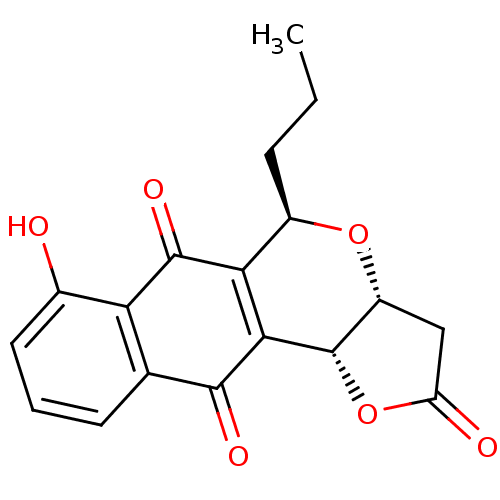
(Frenolicin B)Show SMILES CCC[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1c(O)cccc1C2=O |r,c:12| Show InChI InChI=1S/C18H16O6/c1-2-4-10-14-15(18-11(23-10)7-12(20)24-18)16(21)8-5-3-6-9(19)13(8)17(14)22/h3,5-6,10-11,18-19H,2,4,7H2,1H3/t10-,11-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 using biotinylated GSKalpha peptide (SGRARTSSFA) as a substrate after 1 hr |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM15015
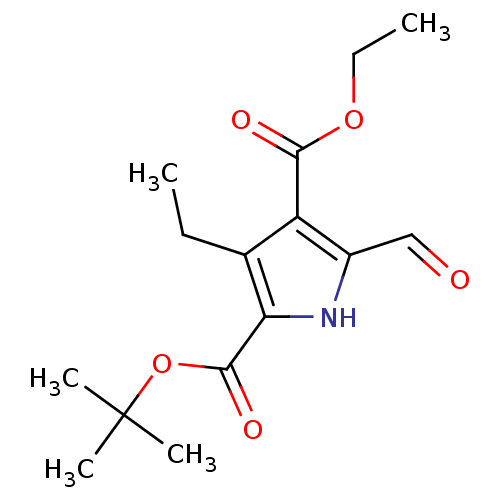
(2-tert-Butyl 4-Ethyl 3-Ethyl-5-formyl-1H-pyrrole-2...)Show InChI InChI=1S/C15H21NO5/c1-6-9-11(13(18)20-7-2)10(8-17)16-12(9)14(19)21-15(3,4)5/h8,16H,6-7H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 4
(Homo sapiens (Human)) | BDBM50336457

((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...)Show SMILES COc1cc(O)c2c(CCCC(=O)C(=O)C(=O)CCC[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h9-11,22H,3-8H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of MKK4 |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50403061

(CHEMBL2216823 | US9353116, 9)Show SMILES CC(C)n1nc(Cc2cccc(NS(=O)(=O)C=C)c2)c2c(N)ncnc12 Show InChI InChI=1S/C17H20N6O2S/c1-4-26(24,25)22-13-7-5-6-12(8-13)9-14-15-16(18)19-10-20-17(15)23(21-14)11(2)3/h4-8,10-11,22H,1,9H2,2-3H3,(H2,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of chicken c-Src using IYGEFKKK peptide as a substrate and [32P]ATP by fluorescence analysis |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM4583

((2E)-N-{4-[(3-bromophenyl)amino]quinazolin-6-yl}-4...)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1 Show InChI InChI=1S/C20H20BrN5O/c1-26(2)10-4-7-19(27)24-16-8-9-18-17(12-16)20(23-13-22-18)25-15-6-3-5-14(21)11-15/h3-9,11-13H,10H2,1-2H3,(H,24,27)(H,22,23,25)/b7-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of chicken c-Src |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50307935
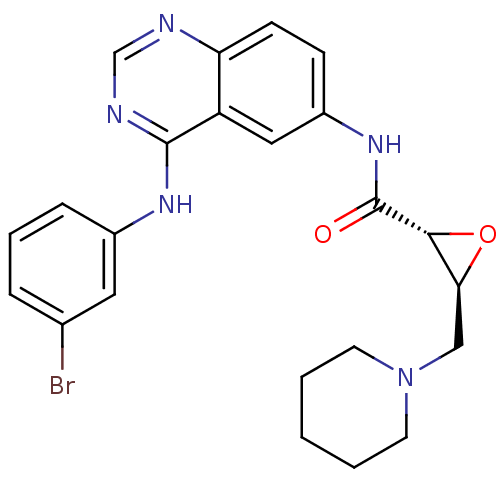
((2R,3S)-N-(4-(3-Bromoanilino)quinazolin-6-yl)-3-(p...)Show SMILES Brc1cccc(Nc2ncnc3ccc(NC(=O)[C@@H]4O[C@H]4CN4CCCCC4)cc23)c1 |r| Show InChI InChI=1S/C23H24BrN5O2/c24-15-5-4-6-16(11-15)27-22-18-12-17(7-8-19(18)25-14-26-22)28-23(30)21-20(31-21)13-29-9-2-1-3-10-29/h4-8,11-12,14,20-21H,1-3,9-10,13H2,(H,28,30)(H,25,26,27)/t20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of chicken c-Src |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of chicken c-Src |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM4566

(4-anilinoquinazoline deriv. 1 | CHEMBL91867 | N-{4...)Show InChI InChI=1S/C18H13BrN4O/c1-2-4-17(24)22-14-7-8-16-15(10-14)18(21-11-20-16)23-13-6-3-5-12(19)9-13/h3,5-11H,1H3,(H,22,24)(H,20,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of chicken c-Src |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data