

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387125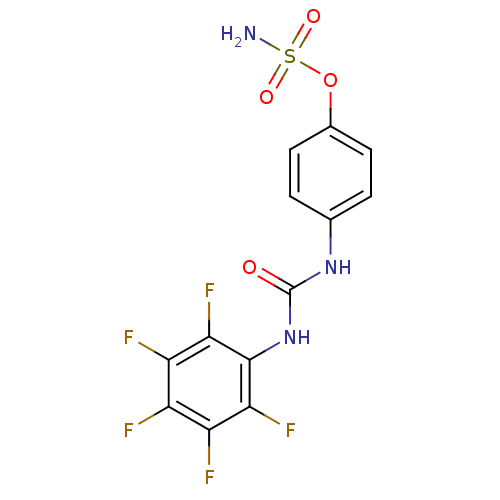 (4-ureidophenyl sulfamate ring derivative 3j | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387131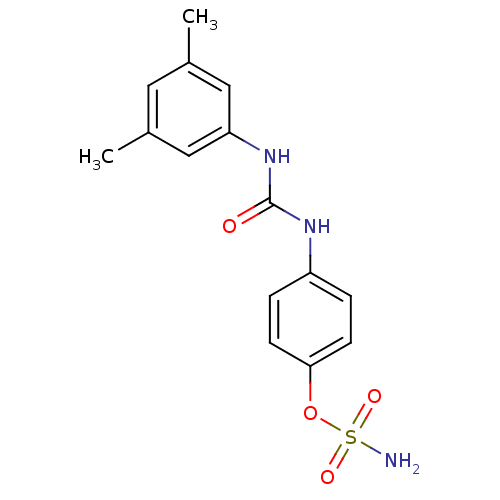 (4-ureidophenyl sulfamate ring derivative 3p | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387129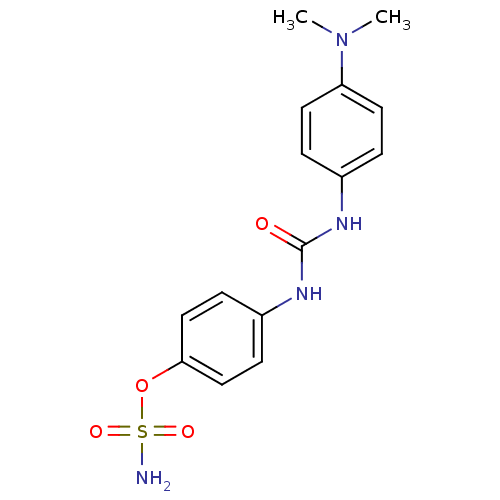 (4-ureidophenyl sulfamate ring derivative 3n | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387117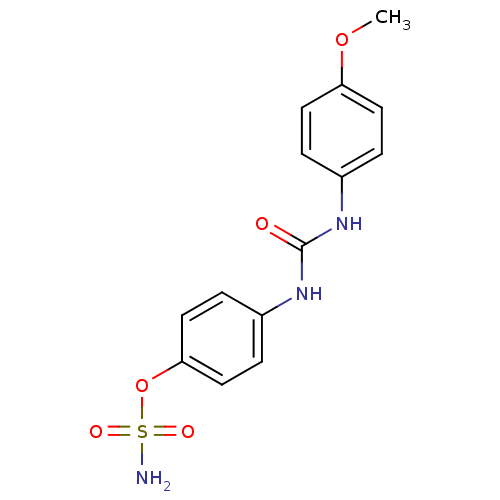 (4-ureidophenyl sulfamate ring derivative 3g | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387128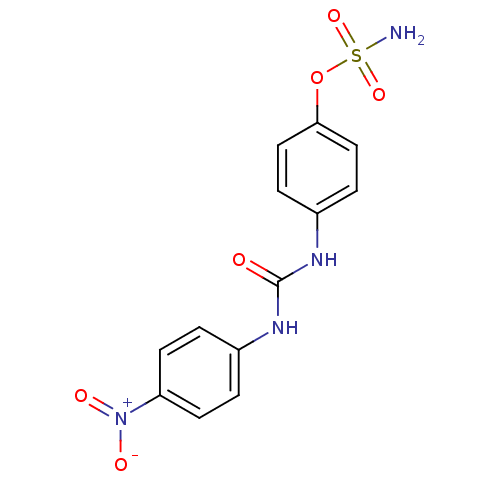 (4-ureidophenyl sulfamate ring derivative 3m | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387116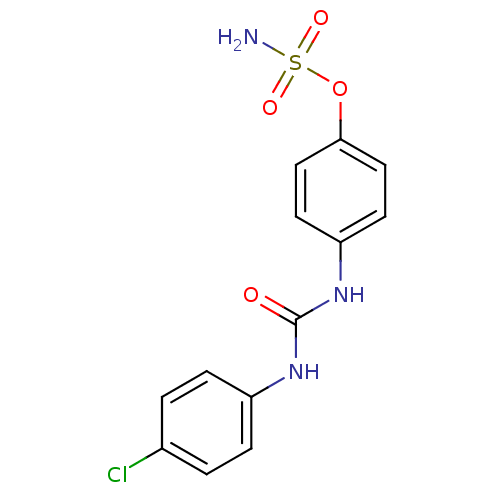 (4-ureidophenyl sulfamate ring derivative 3x | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387125 (4-ureidophenyl sulfamate ring derivative 3j | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387128 (4-ureidophenyl sulfamate ring derivative 3m | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387127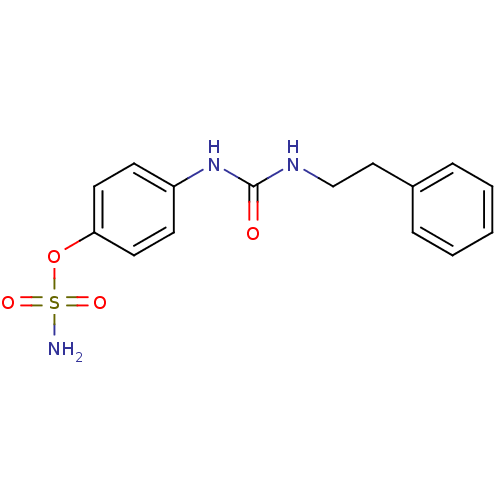 (4-ureidophenyl sulfamate ring derivative 3l | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387131 (4-ureidophenyl sulfamate ring derivative 3p | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387124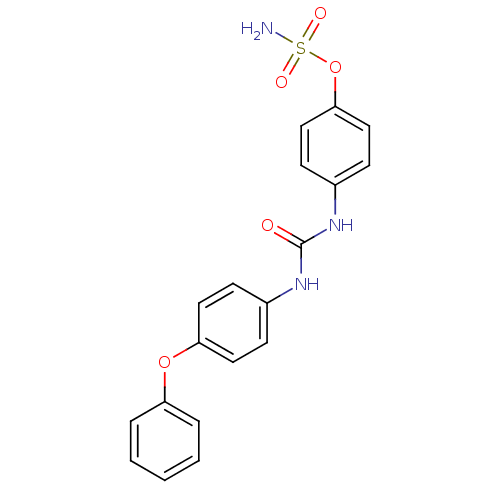 (4-ureidophenyl sulfamate ring derivative 3i | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387115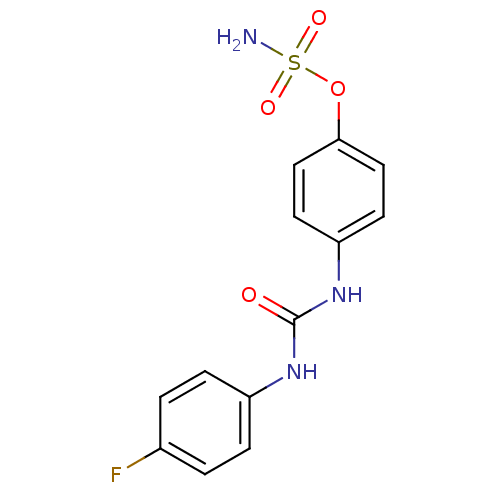 (CHEMBL2047797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387129 (4-ureidophenyl sulfamate ring derivative 3n | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387116 (4-ureidophenyl sulfamate ring derivative 3x | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50391384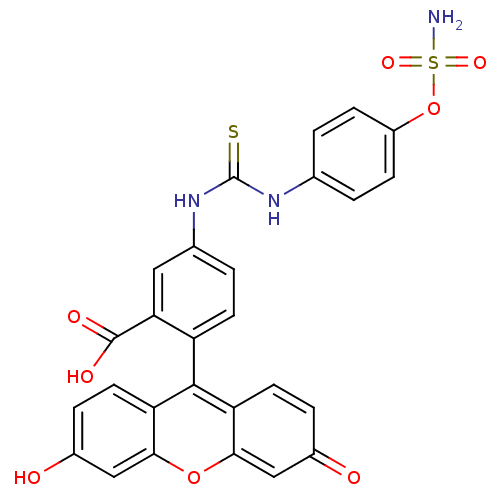 (CHEMBL2148104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387115 (CHEMBL2047797) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50391385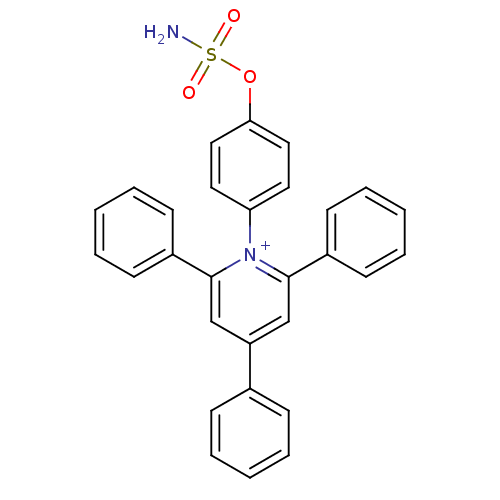 (CHEMBL2148105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387117 (4-ureidophenyl sulfamate ring derivative 3g | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387127 (4-ureidophenyl sulfamate ring derivative 3l | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50391384 (CHEMBL2148104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387124 (4-ureidophenyl sulfamate ring derivative 3i | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA9 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50391385 (CHEMBL2148105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387125 (4-ureidophenyl sulfamate ring derivative 3j | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387127 (4-ureidophenyl sulfamate ring derivative 3l | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387115 (CHEMBL2047797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387116 (4-ureidophenyl sulfamate ring derivative 3x | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387124 (4-ureidophenyl sulfamate ring derivative 3i | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 319 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387129 (4-ureidophenyl sulfamate ring derivative 3n | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387117 (4-ureidophenyl sulfamate ring derivative 3g | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 413 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387128 (4-ureidophenyl sulfamate ring derivative 3m | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387131 (4-ureidophenyl sulfamate ring derivative 3p | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 546 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50391384 (CHEMBL2148104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50391385 (CHEMBL2148105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA2 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387128 (4-ureidophenyl sulfamate ring derivative 3m | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387117 (4-ureidophenyl sulfamate ring derivative 3g | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387115 (CHEMBL2047797) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387116 (4-ureidophenyl sulfamate ring derivative 3x | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387125 (4-ureidophenyl sulfamate ring derivative 3j | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387124 (4-ureidophenyl sulfamate ring derivative 3i | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387129 (4-ureidophenyl sulfamate ring derivative 3n | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387127 (4-ureidophenyl sulfamate ring derivative 3l | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387131 (4-ureidophenyl sulfamate ring derivative 3p | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50391384 (CHEMBL2148104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50391385 (CHEMBL2148105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic CA1 preincubated for 15 mins by stopped-flow CO2 hydration method | J Med Chem 55: 5591-600 (2012) Article DOI: 10.1021/jm300529u BindingDB Entry DOI: 10.7270/Q23R0TZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||