 Found 121 hits of Enzyme Inhibition Constant Data
Found 121 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390392

(CHEMBL2070942)Show SMILES CC(C)(C)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H33N5O2S/c1-22(2,3)18-14-31-21(25-18)28-12-10-27(11-13-28)20(30)17-7-5-4-6-16(17)19(29)26-23(15-24)8-9-23/h14,16-17H,4-13H2,1-3H3,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390403

(CHEMBL2071098)Show SMILES CC1(C)COCc2sc(nc12)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H33N5O3S/c1-23(2)15-32-13-18-19(23)26-22(33-18)29-11-9-28(10-12-29)21(31)17-6-4-3-5-16(17)20(30)27-24(14-25)7-8-24/h16-17H,3-13,15H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390401
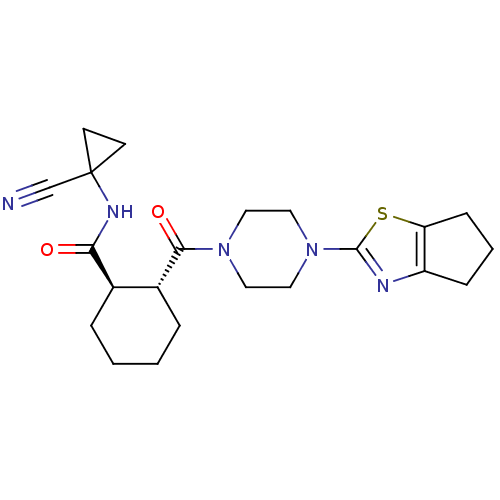
(CHEMBL2071096)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCCc2s1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(8-9-22)25-19(28)15-4-1-2-5-16(15)20(29)26-10-12-27(13-11-26)21-24-17-6-3-7-18(17)30-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390404

(CHEMBL2070936)Show SMILES Fc1ccc(cc1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C22H27FN4O2/c23-16-5-7-17(8-6-16)26-11-13-27(14-12-26)21(29)19-4-2-1-3-18(19)20(28)25-22(15-24)9-10-22/h5-8,18-19H,1-4,9-14H2,(H,25,28)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
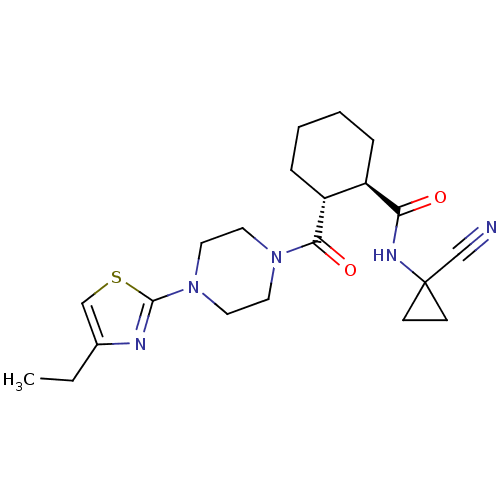
Cathepsin K
(Homo sapiens (Human)) | BDBM50390406
(CHEMBL2069324)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O2S/c26-17-25(10-11-25)28-22(31)19-8-4-5-9-20(19)23(32)29-12-14-30(15-13-29)24-27-21(16-33-24)18-6-2-1-3-7-18/h1-3,6-7,16,19-20H,4-5,8-15H2,(H,28,31)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390405

(CHEMBL2070951)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cnccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-13-24-8-5-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Canis familiaris) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of dog recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent reson... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
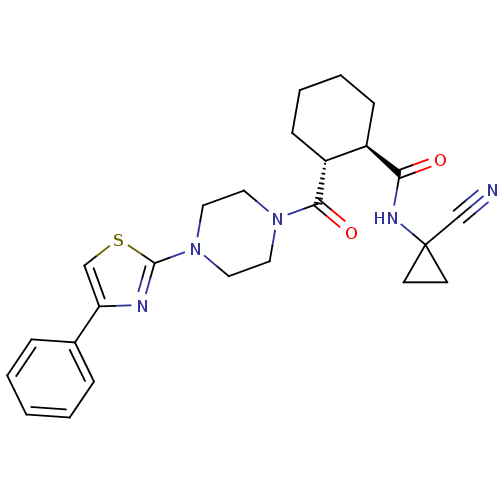
Cathepsin K
(Homo sapiens (Human)) | BDBM50390387
(CHEMBL2070941)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)C1CC1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(7-8-22)25-19(28)16-3-1-2-4-17(16)20(29)26-9-11-27(12-10-26)21-24-18(13-30-21)15-5-6-15/h13,15-17H,1-12H2,(H,25,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390388

(CHEMBL2070939)Show SMILES Cc1nc(sc1C)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-14-15(2)29-20(23-14)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(13-22)7-8-21/h16-17H,3-12H2,1-2H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
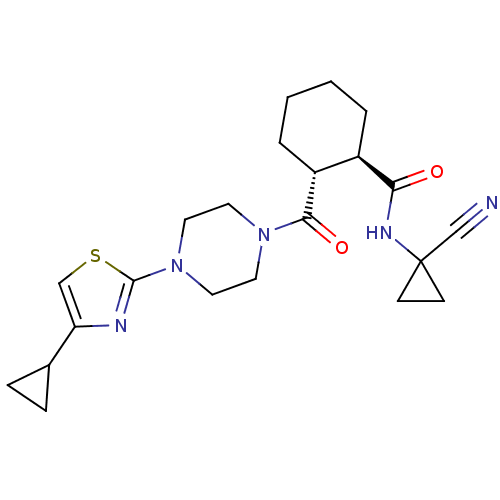
Cathepsin K
(Homo sapiens (Human)) | BDBM50390389
(CHEMBL2070940)Show SMILES CCc1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-2-15-13-29-20(23-15)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(14-22)7-8-21/h13,16-17H,2-12H2,1H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390402
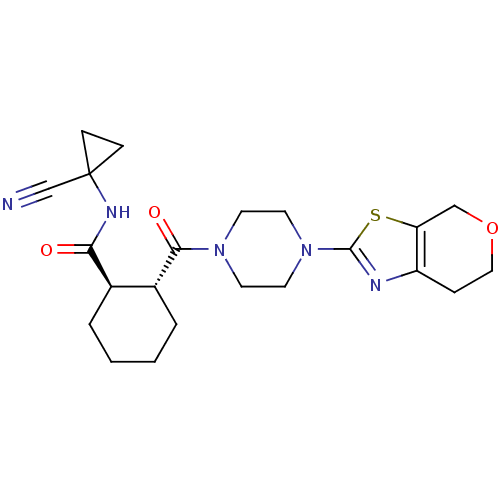
(CHEMBL2071097)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCOCc2s1 |r| Show InChI InChI=1S/C22H29N5O3S/c23-14-22(6-7-22)25-19(28)15-3-1-2-4-16(15)20(29)26-8-10-27(11-9-26)21-24-17-5-12-30-13-18(17)31-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390394

(CHEMBL2070944)Show SMILES Cc1sc(nc1C(F)(F)F)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H26F3N5O2S/c1-13-16(21(22,23)24)26-19(32-13)29-10-8-28(9-11-29)18(31)15-5-3-2-4-14(15)17(30)27-20(12-25)6-7-20/h14-15H,2-11H2,1H3,(H,27,30)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390390

(CHEMBL2070938)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nccs1 |r| Show InChI InChI=1S/C19H25N5O2S/c20-13-19(5-6-19)22-16(25)14-3-1-2-4-15(14)17(26)23-8-10-24(11-9-23)18-21-7-12-27-18/h7,12,14-15H,1-6,8-11H2,(H,22,25)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390395

(CHEMBL2070945)Show SMILES FC(F)(F)c1nc(sc1Cl)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H23ClF3N5O2S/c21-15-14(20(22,23)24)26-18(32-15)29-9-7-28(8-10-29)17(31)13-4-2-1-3-12(13)16(30)27-19(11-25)5-6-19/h12-13H,1-10H2,(H,27,30)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390399

(CHEMBL2070950)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccncc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-5-8-24-13-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390400

(CHEMBL2071095)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ncccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-19(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-18-17(31-21)6-3-9-24-18/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390393
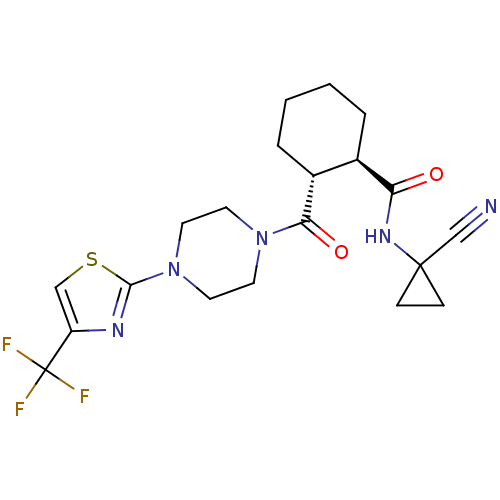
(CHEMBL2070943)Show SMILES FC(F)(F)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H24F3N5O2S/c21-20(22,23)15-11-31-18(25-15)28-9-7-27(8-10-28)17(30)14-4-2-1-3-13(14)16(29)26-19(12-24)5-6-19/h11,13-14H,1-10H2,(H,26,29)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390407

(CHEMBL2070946)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1ncc(s1)C#N |r| Show InChI InChI=1S/C20H24N6O2S/c21-11-14-12-23-19(29-14)26-9-7-25(8-10-26)18(28)16-4-2-1-3-15(16)17(27)24-20(13-22)5-6-20/h12,15-16H,1-10H2,(H,24,27)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390404

(CHEMBL2070936)Show SMILES Fc1ccc(cc1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C22H27FN4O2/c23-16-5-7-17(8-6-16)26-11-13-27(14-12-26)21(29)19-4-2-1-3-18(19)20(28)25-22(15-24)9-10-22/h5-8,18-19H,1-4,9-14H2,(H,25,28)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390404

(CHEMBL2070936)Show SMILES Fc1ccc(cc1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C22H27FN4O2/c23-16-5-7-17(8-6-16)26-11-13-27(14-12-26)21(29)19-4-2-1-3-18(19)20(28)25-22(15-24)9-10-22/h5-8,18-19H,1-4,9-14H2,(H,25,28)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390392

(CHEMBL2070942)Show SMILES CC(C)(C)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H33N5O2S/c1-22(2,3)18-14-31-21(25-18)28-12-10-27(11-13-28)20(30)17-7-5-4-6-16(17)19(29)26-23(15-24)8-9-23/h14,16-17H,4-13H2,1-3H3,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390406

(CHEMBL2069324)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O2S/c26-17-25(10-11-25)28-22(31)19-8-4-5-9-20(19)23(32)29-12-14-30(15-13-29)24-27-21(16-33-24)18-6-2-1-3-7-18/h1-3,6-7,16,19-20H,4-5,8-15H2,(H,28,31)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390395

(CHEMBL2070945)Show SMILES FC(F)(F)c1nc(sc1Cl)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H23ClF3N5O2S/c21-15-14(20(22,23)24)26-18(32-15)29-9-7-28(8-10-29)17(31)13-4-2-1-3-12(13)16(30)27-19(11-25)5-6-19/h12-13H,1-10H2,(H,27,30)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390390

(CHEMBL2070938)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nccs1 |r| Show InChI InChI=1S/C19H25N5O2S/c20-13-19(5-6-19)22-16(25)14-3-1-2-4-15(14)17(26)23-8-10-24(11-9-23)18-21-7-12-27-18/h7,12,14-15H,1-6,8-11H2,(H,22,25)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390393

(CHEMBL2070943)Show SMILES FC(F)(F)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H24F3N5O2S/c21-20(22,23)15-11-31-18(25-15)28-9-7-27(8-10-28)17(30)14-4-2-1-3-13(14)16(29)26-19(12-24)5-6-19/h11,13-14H,1-10H2,(H,26,29)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390400

(CHEMBL2071095)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ncccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-19(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-18-17(31-21)6-3-9-24-18/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390401

(CHEMBL2071096)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCCc2s1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(8-9-22)25-19(28)15-4-1-2-5-16(15)20(29)26-10-12-27(13-11-26)21-24-17-6-3-7-18(17)30-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390394

(CHEMBL2070944)Show SMILES Cc1sc(nc1C(F)(F)F)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H26F3N5O2S/c1-13-16(21(22,23)24)26-19(32-13)29-10-8-28(9-11-29)18(31)15-5-3-2-4-14(15)17(30)27-20(12-25)6-7-20/h14-15H,2-11H2,1H3,(H,27,30)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390399

(CHEMBL2070950)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccncc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-5-8-24-13-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390403

(CHEMBL2071098)Show SMILES CC1(C)COCc2sc(nc12)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H33N5O3S/c1-23(2)15-32-13-18-19(23)26-22(33-18)29-11-9-28(10-12-29)21(31)17-6-4-3-5-16(17)20(30)27-24(14-25)7-8-24/h16-17H,3-13,15H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390405

(CHEMBL2070951)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cnccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-13-24-8-5-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390402

(CHEMBL2071097)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCOCc2s1 |r| Show InChI InChI=1S/C22H29N5O3S/c23-14-22(6-7-22)25-19(28)15-3-1-2-4-16(15)20(29)26-8-10-27(11-9-26)21-24-17-5-12-30-13-18(17)31-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390403

(CHEMBL2071098)Show SMILES CC1(C)COCc2sc(nc12)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H33N5O3S/c1-23(2)15-32-13-18-19(23)26-22(33-18)29-11-9-28(10-12-29)21(31)17-6-4-3-5-16(17)20(30)27-24(14-25)7-8-24/h16-17H,3-13,15H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390388

(CHEMBL2070939)Show SMILES Cc1nc(sc1C)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-14-15(2)29-20(23-14)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(13-22)7-8-21/h16-17H,3-12H2,1-2H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390388

(CHEMBL2070939)Show SMILES Cc1nc(sc1C)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-14-15(2)29-20(23-14)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(13-22)7-8-21/h16-17H,3-12H2,1-2H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390390

(CHEMBL2070938)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nccs1 |r| Show InChI InChI=1S/C19H25N5O2S/c20-13-19(5-6-19)22-16(25)14-3-1-2-4-15(14)17(26)23-8-10-24(11-9-23)18-21-7-12-27-18/h7,12,14-15H,1-6,8-11H2,(H,22,25)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390392

(CHEMBL2070942)Show SMILES CC(C)(C)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H33N5O2S/c1-22(2,3)18-14-31-21(25-18)28-12-10-27(11-13-28)20(30)17-7-5-4-6-16(17)19(29)26-23(15-24)8-9-23/h14,16-17H,4-13H2,1-3H3,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390402

(CHEMBL2071097)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCOCc2s1 |r| Show InChI InChI=1S/C22H29N5O3S/c23-14-22(6-7-22)25-19(28)15-3-1-2-4-16(15)20(29)26-8-10-27(11-9-26)21-24-17-5-12-30-13-18(17)31-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390405

(CHEMBL2070951)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cnccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-13-24-8-5-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390401

(CHEMBL2071096)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCCc2s1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(8-9-22)25-19(28)15-4-1-2-5-16(15)20(29)26-10-12-27(13-11-26)21-24-17-6-3-7-18(17)30-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390389

(CHEMBL2070940)Show SMILES CCc1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-2-15-13-29-20(23-15)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(14-22)7-8-21/h13,16-17H,2-12H2,1H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390389

(CHEMBL2070940)Show SMILES CCc1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-2-15-13-29-20(23-15)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(14-22)7-8-21/h13,16-17H,2-12H2,1H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390407

(CHEMBL2070946)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1ncc(s1)C#N |r| Show InChI InChI=1S/C20H24N6O2S/c21-11-14-12-23-19(29-14)26-9-7-25(8-10-26)18(28)16-4-2-1-3-15(16)17(27)24-20(13-22)5-6-20/h12,15-16H,1-10H2,(H,24,27)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390387

(CHEMBL2070941)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)C1CC1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(7-8-22)25-19(28)16-3-1-2-4-17(16)20(29)26-9-11-27(12-10-26)21-24-18(13-30-21)15-5-6-15/h13,15-17H,1-12H2,(H,25,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390400

(CHEMBL2071095)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ncccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-19(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-18-17(31-21)6-3-9-24-18/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390399

(CHEMBL2070950)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccncc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-5-8-24-13-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390406

(CHEMBL2069324)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O2S/c26-17-25(10-11-25)28-22(31)19-8-4-5-9-20(19)23(32)29-12-14-30(15-13-29)24-27-21(16-33-24)18-6-2-1-3-7-18/h1-3,6-7,16,19-20H,4-5,8-15H2,(H,28,31)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390394

(CHEMBL2070944)Show SMILES Cc1sc(nc1C(F)(F)F)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H26F3N5O2S/c1-13-16(21(22,23)24)26-19(32-13)29-10-8-28(9-11-29)18(31)15-5-3-2-4-14(15)17(30)27-20(12-25)6-7-20/h14-15H,2-11H2,1H3,(H,27,30)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390395

(CHEMBL2070945)Show SMILES FC(F)(F)c1nc(sc1Cl)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H23ClF3N5O2S/c21-15-14(20(22,23)24)26-18(32-15)29-9-7-28(8-10-29)17(31)13-4-2-1-3-12(13)16(30)27-19(11-25)5-6-19/h12-13H,1-10H2,(H,27,30)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390387

(CHEMBL2070941)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)C1CC1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(7-8-22)25-19(28)16-3-1-2-4-17(16)20(29)26-9-11-27(12-10-26)21-24-18(13-30-21)15-5-6-15/h13,15-17H,1-12H2,(H,25,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390393

(CHEMBL2070943)Show SMILES FC(F)(F)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H24F3N5O2S/c21-20(22,23)15-11-31-18(25-15)28-9-7-27(8-10-28)17(30)14-4-2-1-3-13(14)16(29)26-19(12-24)5-6-19/h11,13-14H,1-10H2,(H,26,29)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390392

(CHEMBL2070942)Show SMILES CC(C)(C)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H33N5O2S/c1-22(2,3)18-14-31-21(25-18)28-12-10-27(11-13-28)20(30)17-7-5-4-6-16(17)19(29)26-23(15-24)8-9-23/h14,16-17H,4-13H2,1-3H3,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390394

(CHEMBL2070944)Show SMILES Cc1sc(nc1C(F)(F)F)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H26F3N5O2S/c1-13-16(21(22,23)24)26-19(32-13)29-10-8-28(9-11-29)18(31)15-5-3-2-4-14(15)17(30)27-20(12-25)6-7-20/h14-15H,2-11H2,1H3,(H,27,30)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390393

(CHEMBL2070943)Show SMILES FC(F)(F)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H24F3N5O2S/c21-20(22,23)15-11-31-18(25-15)28-9-7-27(8-10-28)17(30)14-4-2-1-3-13(14)16(29)26-19(12-24)5-6-19/h11,13-14H,1-10H2,(H,26,29)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390395

(CHEMBL2070945)Show SMILES FC(F)(F)c1nc(sc1Cl)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H23ClF3N5O2S/c21-15-14(20(22,23)24)26-18(32-15)29-9-7-28(8-10-29)17(31)13-4-2-1-3-12(13)16(30)27-19(11-25)5-6-19/h12-13H,1-10H2,(H,27,30)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390406

(CHEMBL2069324)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O2S/c26-17-25(10-11-25)28-22(31)19-8-4-5-9-20(19)23(32)29-12-14-30(15-13-29)24-27-21(16-33-24)18-6-2-1-3-7-18/h1-3,6-7,16,19-20H,4-5,8-15H2,(H,28,31)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B using Z-Arg-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50390391

(CHEMBL2070937)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H27N5O2S/c24-15-23(9-10-23)26-20(29)16-5-1-2-6-17(16)21(30)27-11-13-28(14-12-27)22-25-18-7-3-4-8-19(18)31-22/h3-4,7-8,16-17H,1-2,5-6,9-14H2,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390404

(CHEMBL2070936)Show SMILES Fc1ccc(cc1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C22H27FN4O2/c23-16-5-7-17(8-6-16)26-11-13-27(14-12-26)21(29)19-4-2-1-3-18(19)20(28)25-22(15-24)9-10-22/h5-8,18-19H,1-4,9-14H2,(H,25,28)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390401

(CHEMBL2071096)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCCc2s1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(8-9-22)25-19(28)15-4-1-2-5-16(15)20(29)26-10-12-27(13-11-26)21-24-17-6-3-7-18(17)30-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390395

(CHEMBL2070945)Show SMILES FC(F)(F)c1nc(sc1Cl)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H23ClF3N5O2S/c21-15-14(20(22,23)24)26-18(32-15)29-9-7-28(8-10-29)17(31)13-4-2-1-3-12(13)16(30)27-19(11-25)5-6-19/h12-13H,1-10H2,(H,27,30)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390402

(CHEMBL2071097)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCOCc2s1 |r| Show InChI InChI=1S/C22H29N5O3S/c23-14-22(6-7-22)25-19(28)15-3-1-2-4-16(15)20(29)26-8-10-27(11-9-26)21-24-17-5-12-30-13-18(17)31-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390399

(CHEMBL2070950)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccncc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-5-8-24-13-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390405

(CHEMBL2070951)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cnccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-13-24-8-5-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390387

(CHEMBL2070941)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)C1CC1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(7-8-22)25-19(28)16-3-1-2-4-17(16)20(29)26-9-11-27(12-10-26)21-24-18(13-30-21)15-5-6-15/h13,15-17H,1-12H2,(H,25,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390388

(CHEMBL2070939)Show SMILES Cc1nc(sc1C)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-14-15(2)29-20(23-14)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(13-22)7-8-21/h16-17H,3-12H2,1-2H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390402

(CHEMBL2071097)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2CCOCc2s1 |r| Show InChI InChI=1S/C22H29N5O3S/c23-14-22(6-7-22)25-19(28)15-3-1-2-4-16(15)20(29)26-8-10-27(11-9-26)21-24-17-5-12-30-13-18(17)31-21/h15-16H,1-13H2,(H,25,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390400

(CHEMBL2071095)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ncccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-19(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-18-17(31-21)6-3-9-24-18/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390387

(CHEMBL2070941)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)C1CC1 |r| Show InChI InChI=1S/C22H29N5O2S/c23-14-22(7-8-22)25-19(28)16-3-1-2-4-17(16)20(29)26-9-11-27(12-10-26)21-24-18(13-30-21)15-5-6-15/h13,15-17H,1-12H2,(H,25,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390389

(CHEMBL2070940)Show SMILES CCc1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-2-15-13-29-20(23-15)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(14-22)7-8-21/h13,16-17H,2-12H2,1H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390403

(CHEMBL2071098)Show SMILES CC1(C)COCc2sc(nc12)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H33N5O3S/c1-23(2)15-32-13-18-19(23)26-22(33-18)29-11-9-28(10-12-29)21(31)17-6-4-3-5-16(17)20(30)27-24(14-25)7-8-24/h16-17H,3-13,15H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390399

(CHEMBL2070950)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ccncc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-5-8-24-13-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390392

(CHEMBL2070942)Show SMILES CC(C)(C)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H33N5O2S/c1-22(2,3)18-14-31-21(25-18)28-12-10-27(11-13-28)20(30)17-7-5-4-6-16(17)19(29)26-23(15-24)8-9-23/h14,16-17H,4-13H2,1-3H3,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390400

(CHEMBL2071095)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2ncccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-19(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-18-17(31-21)6-3-9-24-18/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390388

(CHEMBL2070939)Show SMILES Cc1nc(sc1C)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-14-15(2)29-20(23-14)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(13-22)7-8-21/h16-17H,3-12H2,1-2H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50390396

(CHEMBL2070947)Show SMILES CS(=O)(=O)c1ccc2nc(sc2c1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H29N5O4S2/c1-35(32,33)16-6-7-19-20(14-16)34-23(26-19)29-12-10-28(11-13-29)22(31)18-5-3-2-4-17(18)21(30)27-24(15-25)8-9-24/h6-7,14,17-18H,2-5,8-13H2,1H3,(H,27,30)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Z-Val-Val-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390405

(CHEMBL2070951)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cnccc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(6-7-22)26-19(29)15-3-1-2-4-16(15)20(30)27-9-11-28(12-10-27)21-25-17-13-24-8-5-18(17)31-21/h5,8,13,15-16H,1-4,6-7,9-12H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390404

(CHEMBL2070936)Show SMILES Fc1ccc(cc1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C22H27FN4O2/c23-16-5-7-17(8-6-16)26-11-13-27(14-12-26)21(29)19-4-2-1-3-18(19)20(28)25-22(15-24)9-10-22/h5-8,18-19H,1-4,9-14H2,(H,25,28)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390393

(CHEMBL2070943)Show SMILES FC(F)(F)c1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C20H24F3N5O2S/c21-20(22,23)15-11-31-18(25-15)28-9-7-27(8-10-28)17(30)14-4-2-1-3-13(14)16(29)26-19(12-24)5-6-19/h11,13-14H,1-10H2,(H,26,29)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390398

(CHEMBL2070949)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc2cccnc2s1 |r| Show InChI InChI=1S/C22H26N6O2S/c23-14-22(7-8-22)26-18(29)15-4-1-2-5-16(15)20(30)27-10-12-28(13-11-27)21-25-17-6-3-9-24-19(17)31-21/h3,6,9,15-16H,1-2,4-5,7-8,10-13H2,(H,26,29)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390397

(CHEMBL2070948)Show SMILES CC(C)(C#N)c1cnc(s1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H30N6O2S/c1-22(2,14-24)18-13-26-21(32-18)29-11-9-28(10-12-29)20(31)17-6-4-3-5-16(17)19(30)27-23(15-25)7-8-23/h13,16-17H,3-12H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390390

(CHEMBL2070938)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nccs1 |r| Show InChI InChI=1S/C19H25N5O2S/c20-13-19(5-6-19)22-16(25)14-3-1-2-4-15(14)17(26)23-8-10-24(11-9-23)18-21-7-12-27-18/h7,12,14-15H,1-6,8-11H2,(H,22,25)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50390403

(CHEMBL2071098)Show SMILES CC1(C)COCc2sc(nc12)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H33N5O3S/c1-23(2)15-32-13-18-19(23)26-22(33-18)29-11-9-28(10-12-29)21(31)17-6-4-3-5-16(17)20(30)27-24(14-25)7-8-24/h16-17H,3-13,15H2,1-2H3,(H,27,30)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390394

(CHEMBL2070944)Show SMILES Cc1sc(nc1C(F)(F)F)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H26F3N5O2S/c1-13-16(21(22,23)24)26-19(32-13)29-10-8-28(9-11-29)18(31)15-5-3-2-4-14(15)17(30)27-20(12-25)6-7-20/h14-15H,2-11H2,1H3,(H,27,30)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390406

(CHEMBL2069324)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O2S/c26-17-25(10-11-25)28-22(31)19-8-4-5-9-20(19)23(32)29-12-14-30(15-13-29)24-27-21(16-33-24)18-6-2-1-3-7-18/h1-3,6-7,16,19-20H,4-5,8-15H2,(H,28,31)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390390

(CHEMBL2070938)Show SMILES O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCN(CC1)c1nccs1 |r| Show InChI InChI=1S/C19H25N5O2S/c20-13-19(5-6-19)22-16(25)14-3-1-2-4-15(14)17(26)23-8-10-24(11-9-23)18-21-7-12-27-18/h7,12,14-15H,1-6,8-11H2,(H,22,25)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50390389

(CHEMBL2070940)Show SMILES CCc1csc(n1)N1CCN(CC1)C(=O)[C@@H]1CCCC[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C21H29N5O2S/c1-2-15-13-29-20(23-15)26-11-9-25(10-12-26)19(28)17-6-4-3-5-16(17)18(27)24-21(14-22)7-8-21/h13,16-17H,2-12H2,1H3,(H,24,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-Phe-Arg-AMC as substrate preincubated for 30 mins measured after 1 hr by quenched fluorescent res... |
Bioorg Med Chem Lett 22: 5563-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.012
BindingDB Entry DOI: 10.7270/Q2DF6S86 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data