

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50007674 ((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398926 (CHEMBL2178786) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398918 (CHEMBL2178778) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398928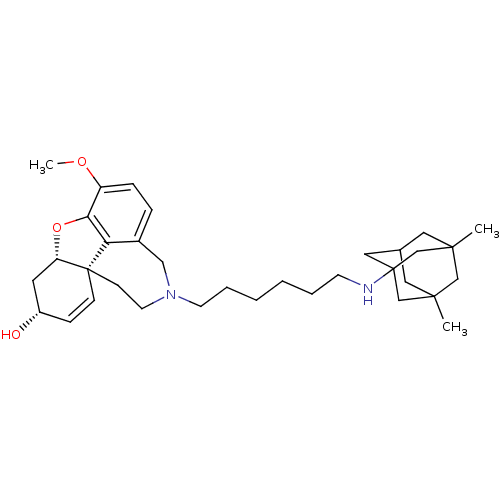 (CHEMBL2178784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398924 (CHEMBL2178788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398915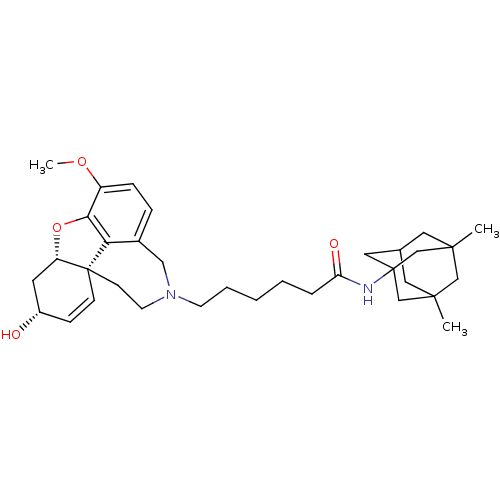 (CHEMBL2178781) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398927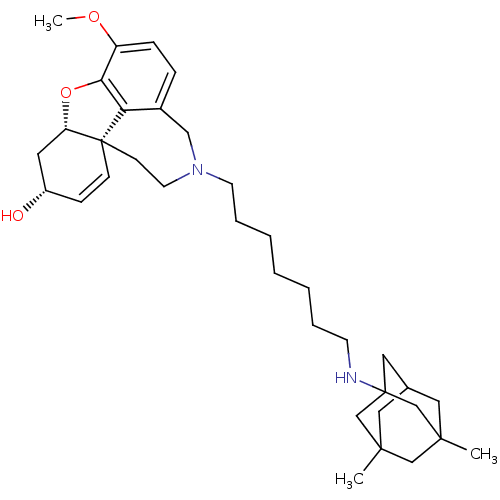 (CHEMBL2178785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398929 (CHEMBL2178783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398930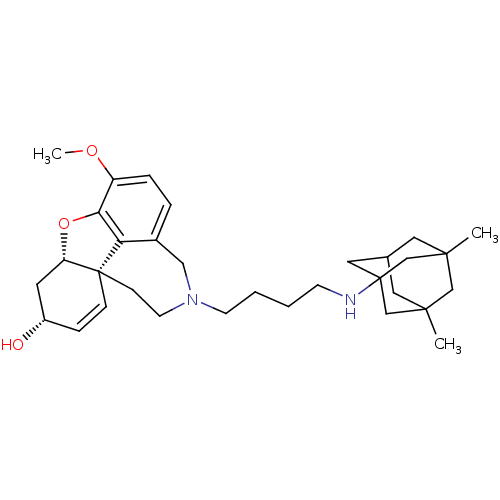 (CHEMBL2178782) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398925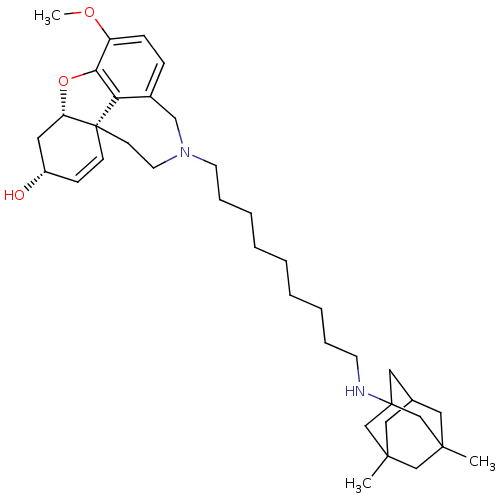 (CHEMBL2178787) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398923 (CHEMBL2178789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398922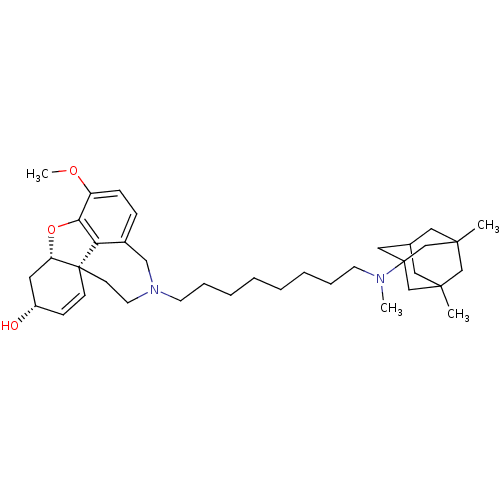 (CHEMBL2178790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398916 (CHEMBL2178780) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398921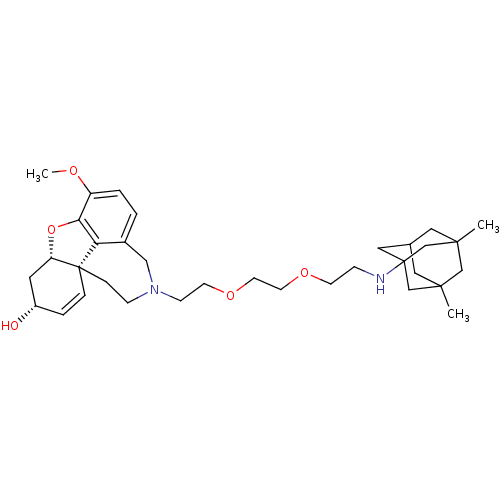 (CHEMBL2178791) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50062599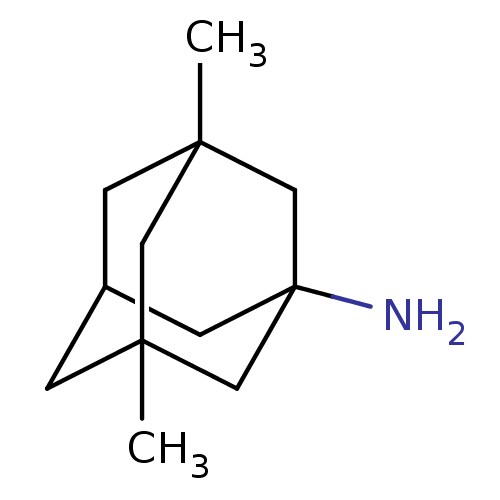 (3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50030386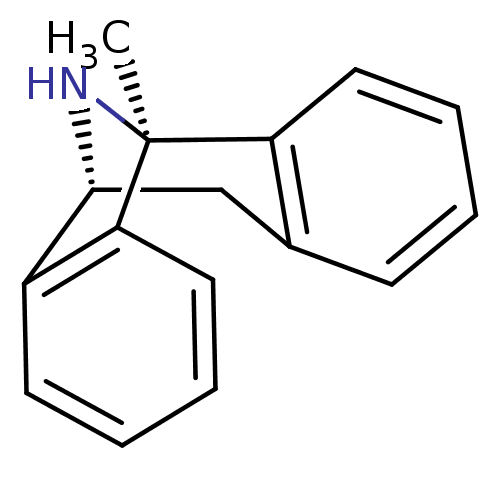 ((1S)-1-methyl-16-azatetracyclo[7.6.1.0^{2,7}.0^{10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398920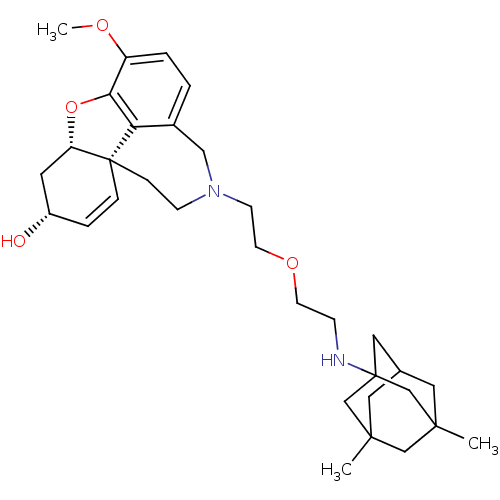 (CHEMBL2178776) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398917 (CHEMBL2178779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50398919 (CHEMBL2178777) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation counting | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398922 (CHEMBL2178790) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398924 (CHEMBL2178788) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398928 (CHEMBL2178784) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398923 (CHEMBL2178789) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398927 (CHEMBL2178785) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398925 (CHEMBL2178787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398929 (CHEMBL2178783) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398926 (CHEMBL2178786) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398915 (CHEMBL2178781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398917 (CHEMBL2178779) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398921 (CHEMBL2178791) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398919 (CHEMBL2178777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398918 (CHEMBL2178778) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398916 (CHEMBL2178780) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398930 (CHEMBL2178782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 696 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398920 (CHEMBL2178776) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||