 Found 92 hits of Enzyme Inhibition Constant Data
Found 92 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK1 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399015
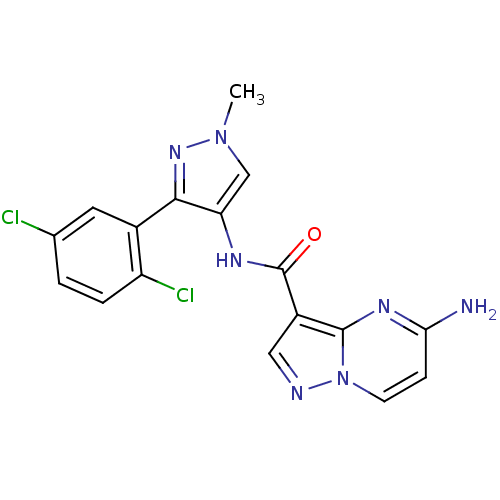
(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399014
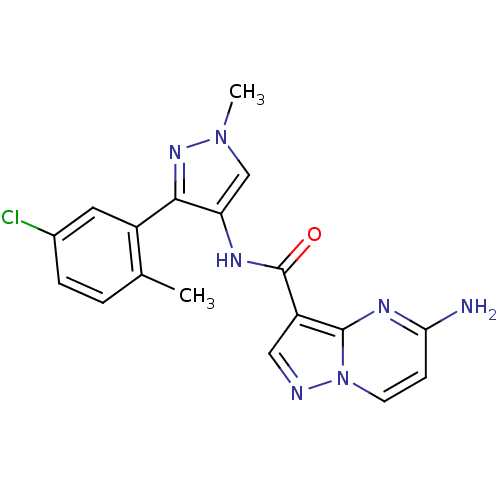
(CHEMBL2178805)Show SMILES Cc1ccc(Cl)cc1-c1nn(C)cc1NC(=O)c1cnn2ccc(N)nc12 Show InChI InChI=1S/C18H16ClN7O/c1-10-3-4-11(19)7-12(10)16-14(9-25(2)24-16)22-18(27)13-8-21-26-6-5-15(20)23-17(13)26/h3-9H,1-2H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399016

(CHEMBL2178803 | US8637526, 225)Show SMILES Cc1ccc(C)c(c1)-c1nn(C)cc1NC(=O)c1cnn2ccc(N)nc12 Show InChI InChI=1S/C19H19N7O/c1-11-4-5-12(2)13(8-11)17-15(10-25(3)24-17)22-19(27)14-9-21-26-7-6-16(20)23-18(14)26/h4-10H,1-3H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399019
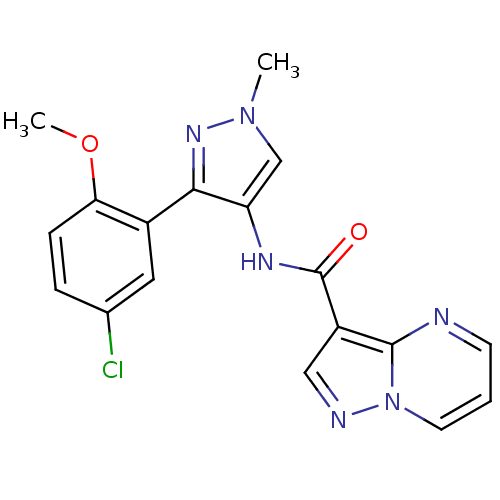
(CHEMBL2178801)Show SMILES COc1ccc(Cl)cc1-c1nn(C)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C18H15ClN6O2/c1-24-10-14(16(23-24)12-8-11(19)4-5-15(12)27-2)22-18(26)13-9-21-25-7-3-6-20-17(13)25/h3-10H,1-2H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399021
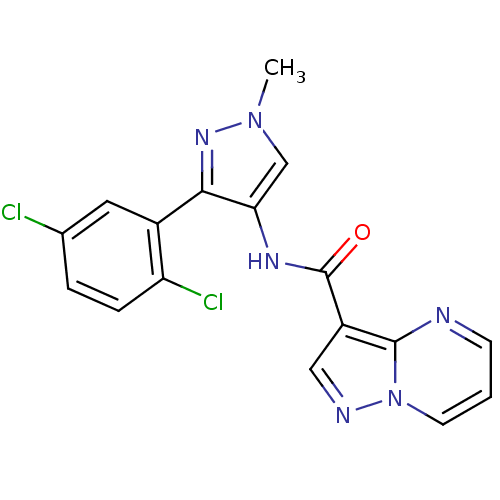
(CHEMBL2178799 | US8999998, 28)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H12Cl2N6O/c1-24-9-14(15(23-24)11-7-10(18)3-4-13(11)19)22-17(26)12-8-21-25-6-2-5-20-16(12)25/h2-9H,1H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified TYK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399020
(CHEMBL2178800)Show SMILES Cc1ccc(Cl)cc1-c1nn(C)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C18H15ClN6O/c1-11-4-5-12(19)8-13(11)16-15(10-24(2)23-16)22-18(26)14-9-21-25-7-3-6-20-17(14)25/h3-10H,1-2H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399009
(CHEMBL2178254)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-24-9-13(15(23-24)10-3-2-4-11(18)7-10)21-17(26)12-8-20-25-6-5-14(19)22-16(12)25/h2-9H,1H3,(H2,19,22)(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399030
(CHEMBL2178258)Show SMILES Cc1nc(c(NC(=O)c2cnn3ccc(N)nc23)s1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6OS/c1-9-21-14(10-3-2-4-11(18)7-10)17(26-9)23-16(25)12-8-20-24-6-5-13(19)22-15(12)24/h2-8H,1H3,(H2,19,22)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399033
(CHEMBL2178255)Show SMILES Nc1ccn2ncc(C(=O)Nc3c[nH]nc3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C16H12ClN7O/c17-10-3-1-2-9(6-10)14-12(8-19-23-14)21-16(25)11-7-20-24-5-4-13(18)22-15(11)24/h1-8H,(H2,18,22)(H,19,23)(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399017
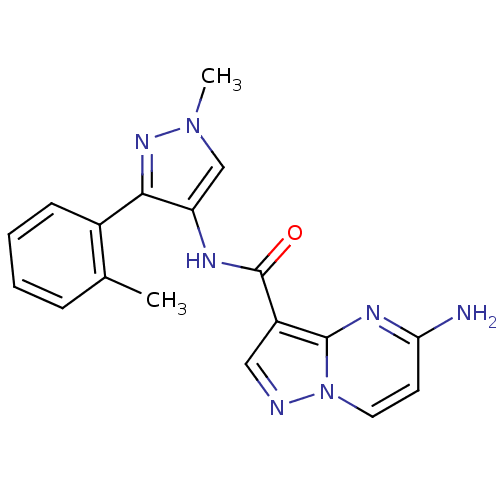
(CHEMBL2178802)Show InChI InChI=1S/C18H17N7O/c1-11-5-3-4-6-12(11)16-14(10-24(2)23-16)21-18(26)13-9-20-25-8-7-15(19)22-17(13)25/h3-10H,1-2H3,(H2,19,22)(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399031
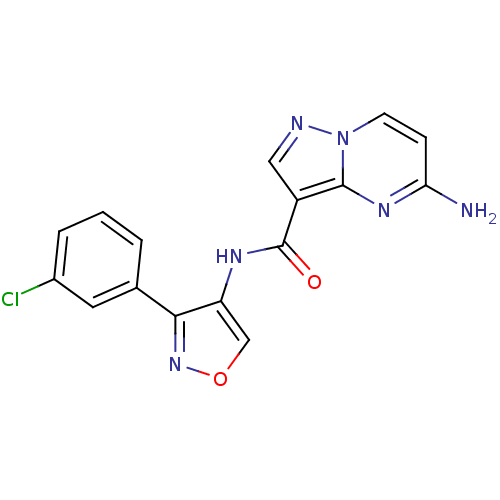
(CHEMBL2178257 | US8637526, 250)Show InChI InChI=1S/C16H11ClN6O2/c17-10-3-1-2-9(6-10)14-12(8-25-22-14)20-16(24)11-7-19-23-5-4-13(18)21-15(11)23/h1-8H,(H2,18,21)(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399022
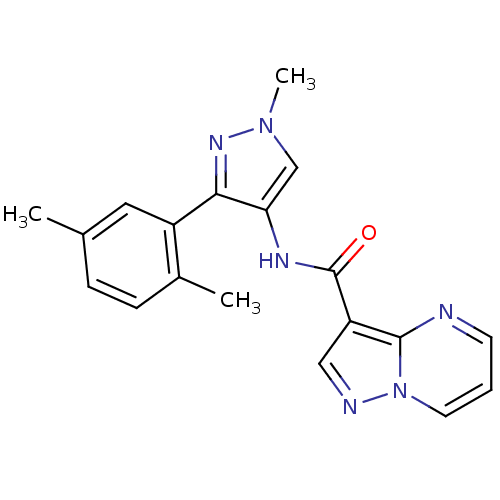
(CHEMBL2178266)Show SMILES Cc1ccc(C)c(c1)-c1nn(C)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C19H18N6O/c1-12-5-6-13(2)14(9-12)17-16(11-24(3)23-17)22-19(26)15-10-21-25-8-4-7-20-18(15)25/h4-11H,1-3H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399037
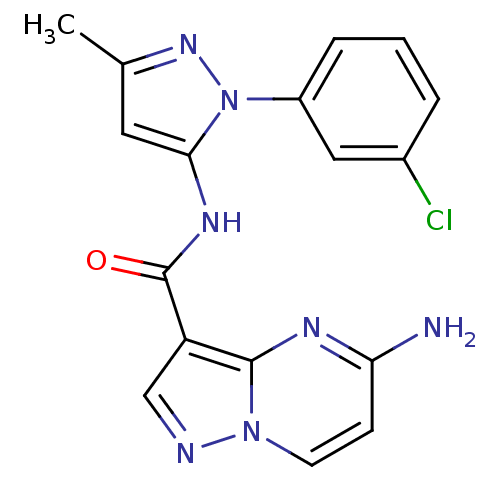
(CHEMBL2178807)Show SMILES Cc1cc(NC(=O)c2cnn3ccc(N)nc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-10-7-15(25(23-10)12-4-2-3-11(18)8-12)22-17(26)13-9-20-24-6-5-14(19)21-16(13)24/h2-9H,1H3,(H2,19,21)(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399034
(CHEMBL2178253)Show InChI InChI=1S/C16H11ClN6O/c17-11-4-1-3-10(7-11)14-13(9-19-22-14)21-16(24)12-8-20-23-6-2-5-18-15(12)23/h1-9H,(H,19,22)(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399010
(CHEMBL2178252)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(15(22-23)11-4-2-5-12(18)8-11)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK3 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399036

(CHEMBL2178247)Show SMILES CNc1ccn2ncc(C(=O)Nc3cc(C)nn3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C18H16ClN7O/c1-11-8-16(26(24-11)13-5-3-4-12(19)9-13)23-18(27)14-10-21-25-7-6-15(20-2)22-17(14)25/h3-10H,1-2H3,(H,20,22)(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399018

(CHEMBL2177122)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1c(Cl)cccc1Cl |(4.01,-28.58,;5.47,-28.11,;5.95,-26.65,;7.5,-26.65,;8.41,-25.41,;7.78,-24,;6.25,-23.83,;8.69,-22.76,;8.22,-21.29,;9.46,-20.38,;10.71,-21.29,;12.22,-20.97,;13.26,-22.13,;12.78,-23.6,;11.26,-23.91,;10.23,-22.76,;7.96,-28.12,;6.71,-29.02,;9.43,-28.6,;10.57,-27.58,;10.25,-26.08,;12.03,-28.06,;12.35,-29.57,;11.19,-30.6,;9.73,-30.11,;8.58,-31.14,)| Show InChI InChI=1S/C17H12Cl2N6O/c1-24-9-13(15(23-24)14-11(18)4-2-5-12(14)19)22-17(26)10-8-21-25-7-3-6-20-16(10)25/h2-9H,1H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399028
(CHEMBL2178260)Show SMILES OCCn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C18H15ClN6O2/c19-13-4-1-3-12(9-13)16-15(11-24(23-16)7-8-26)22-18(27)14-10-21-25-6-2-5-20-17(14)25/h1-6,9-11,26H,7-8H2,(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399035
(CHEMBL2178249)Show InChI InChI=1S/C18H12ClN5O/c19-13-4-1-3-12(9-13)14-10-20-7-5-16(14)23-18(25)15-11-22-24-8-2-6-21-17(15)24/h1-11H,(H,20,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399012
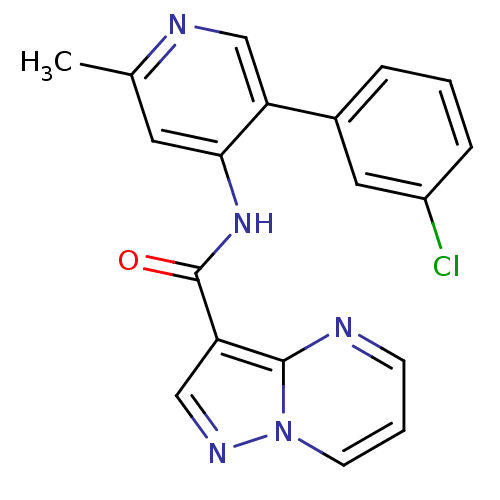
(CHEMBL2178250)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)c(cn1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H14ClN5O/c1-12-8-17(15(10-22-12)13-4-2-5-14(20)9-13)24-19(26)16-11-23-25-7-3-6-21-18(16)25/h2-11H,1H3,(H,22,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399038
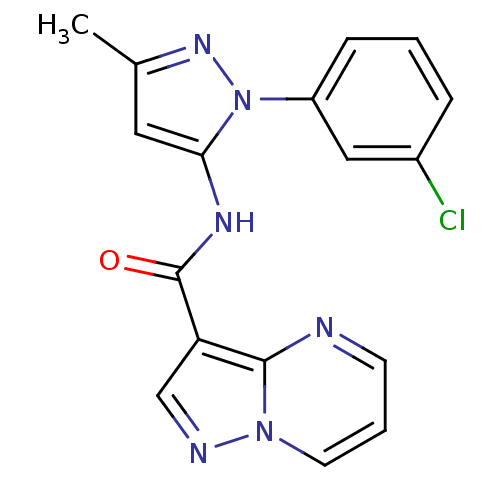
(CHEMBL2178806)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-11-8-15(24(22-11)13-5-2-4-12(18)9-13)21-17(25)14-10-20-23-7-3-6-19-16(14)23/h2-10H,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399027
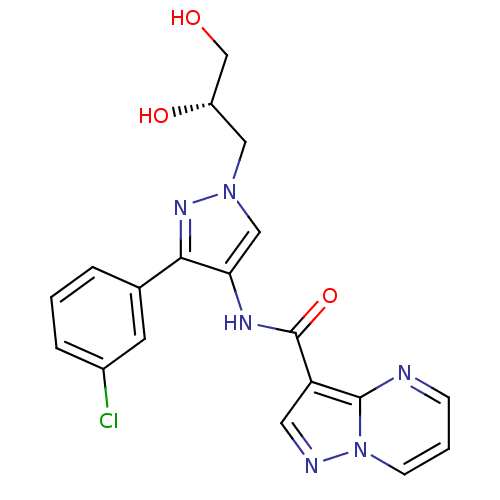
(CHEMBL2178261)Show SMILES OC[C@@H](O)Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C19H17ClN6O3/c20-13-4-1-3-12(7-13)17-16(10-25(24-17)9-14(28)11-27)23-19(29)15-8-22-26-6-2-5-21-18(15)26/h1-8,10,14,27-28H,9,11H2,(H,23,29)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399013
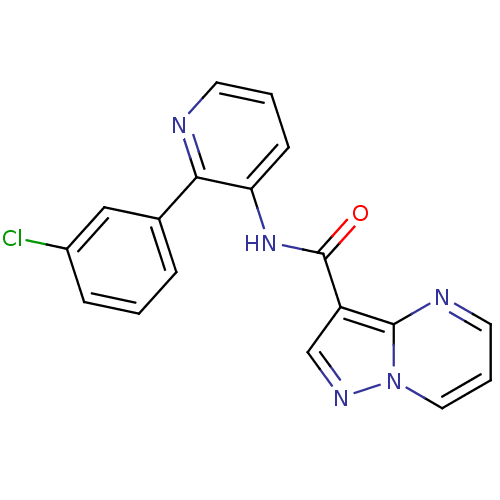
(CHEMBL2178248)Show InChI InChI=1S/C18H12ClN5O/c19-13-5-1-4-12(10-13)16-15(6-2-7-20-16)23-18(25)14-11-22-24-9-3-8-21-17(14)24/h1-11H,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399025
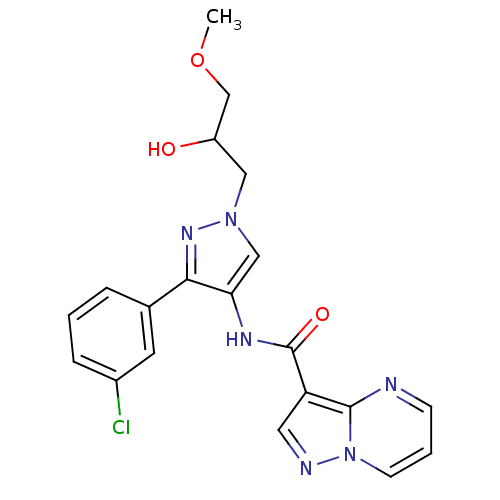
(CHEMBL2178263)Show SMILES COCC(O)Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C20H19ClN6O3/c1-30-12-15(28)10-26-11-17(18(25-26)13-4-2-5-14(21)8-13)24-20(29)16-9-23-27-7-3-6-22-19(16)27/h2-9,11,15,28H,10,12H2,1H3,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399032
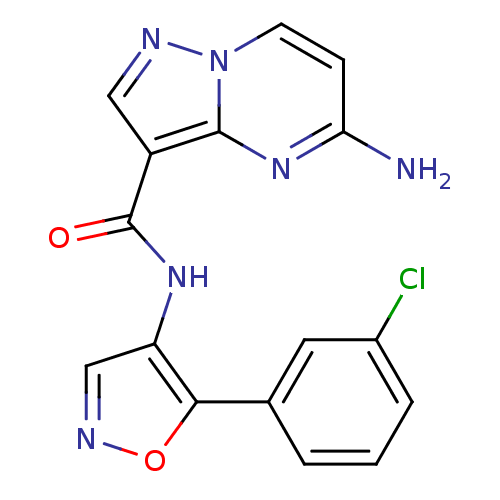
(CHEMBL2178256)Show InChI InChI=1S/C16H11ClN6O2/c17-10-3-1-2-9(6-10)14-12(8-20-25-14)21-16(24)11-7-19-23-5-4-13(18)22-15(11)23/h1-8H,(H2,18,22)(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399026
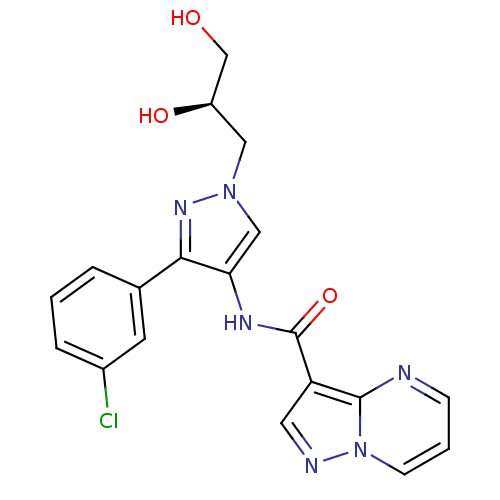
(CHEMBL2178262)Show SMILES OC[C@H](O)Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C19H17ClN6O3/c20-13-4-1-3-12(7-13)17-16(10-25(24-17)9-14(28)11-27)23-19(29)15-8-22-26-6-2-5-21-18(15)26/h1-8,10,14,27-28H,9,11H2,(H,23,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399024
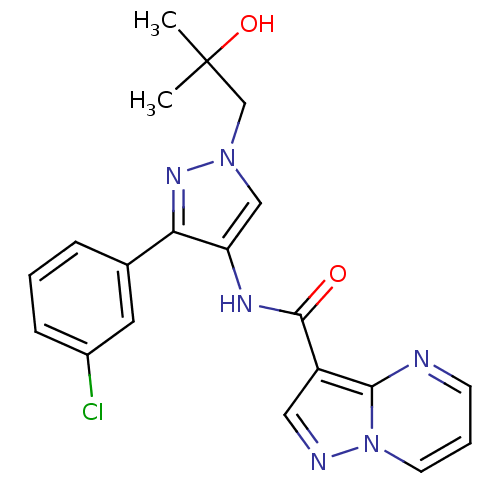
(CHEMBL2178264)Show SMILES CC(C)(O)Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C20H19ClN6O2/c1-20(2,29)12-26-11-16(17(25-26)13-5-3-6-14(21)9-13)24-19(28)15-10-23-27-8-4-7-22-18(15)27/h3-11,29H,12H2,1-2H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399029
(CHEMBL2178259)Show SMILES CC(C)n1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H17ClN6O/c1-12(2)26-11-16(17(24-26)13-5-3-6-14(20)9-13)23-19(27)15-10-22-25-8-4-7-21-18(15)25/h3-12H,1-2H3,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399023

(CHEMBL2178265)Show SMILES Clc1cccc(c1)-c1nn(CCN2CCOCC2)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C22H22ClN7O2/c23-17-4-1-3-16(13-17)20-19(15-29(27-20)8-7-28-9-11-32-12-10-28)26-22(31)18-14-25-30-6-2-5-24-21(18)30/h1-6,13-15H,7-12H2,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399011

(CHEMBL2178251)Show SMILES Cn1cc(c(NC(=O)c2cnn3cccnc23)n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(11-4-2-5-12(18)8-11)15(22-23)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6.85 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399014

(CHEMBL2178805)Show SMILES Cc1ccc(Cl)cc1-c1nn(C)cc1NC(=O)c1cnn2ccc(N)nc12 Show InChI InChI=1S/C18H16ClN7O/c1-10-3-4-11(19)7-12(10)16-14(9-25(2)24-16)22-18(27)13-8-21-26-6-5-15(20)23-17(13)26/h3-9H,1-2H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399019

(CHEMBL2178801)Show SMILES COc1ccc(Cl)cc1-c1nn(C)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C18H15ClN6O2/c1-24-10-14(16(23-24)12-8-11(19)4-5-15(12)27-2)22-18(26)13-9-21-25-7-3-6-20-17(13)25/h3-10H,1-2H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399020

(CHEMBL2178800)Show SMILES Cc1ccc(Cl)cc1-c1nn(C)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C18H15ClN6O/c1-11-4-5-12(19)8-13(11)16-15(10-24(2)23-16)22-18(26)14-9-21-25-7-3-6-20-17(14)25/h3-10H,1-2H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399016

(CHEMBL2178803 | US8637526, 225)Show SMILES Cc1ccc(C)c(c1)-c1nn(C)cc1NC(=O)c1cnn2ccc(N)nc12 Show InChI InChI=1S/C19H19N7O/c1-11-4-5-12(2)13(8-11)17-15(10-25(3)24-17)22-19(27)14-9-21-26-7-6-16(20)23-18(14)26/h4-10H,1-3H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399021

(CHEMBL2178799 | US8999998, 28)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H12Cl2N6O/c1-24-9-14(15(23-24)11-7-10(18)3-4-13(11)19)22-17(26)12-8-21-25-6-2-5-20-16(12)25/h2-9H,1H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399022

(CHEMBL2178266)Show SMILES Cc1ccc(C)c(c1)-c1nn(C)cc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C19H18N6O/c1-12-5-6-13(2)14(9-12)17-16(11-24(3)23-17)22-19(26)15-10-21-25-8-4-7-20-18(15)25/h4-11H,1-3H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399017

(CHEMBL2178802)Show InChI InChI=1S/C18H17N7O/c1-11-5-3-4-6-12(11)16-14(10-24(2)23-16)21-18(26)13-9-20-25-8-7-15(19)22-17(13)25/h3-10H,1-2H3,(H2,19,22)(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399035

(CHEMBL2178249)Show InChI InChI=1S/C18H12ClN5O/c19-13-4-1-3-12(9-13)14-10-20-7-5-16(14)23-18(25)15-11-22-24-8-2-6-21-17(15)24/h1-11H,(H,20,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399018

(CHEMBL2177122)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1c(Cl)cccc1Cl |(4.01,-28.58,;5.47,-28.11,;5.95,-26.65,;7.5,-26.65,;8.41,-25.41,;7.78,-24,;6.25,-23.83,;8.69,-22.76,;8.22,-21.29,;9.46,-20.38,;10.71,-21.29,;12.22,-20.97,;13.26,-22.13,;12.78,-23.6,;11.26,-23.91,;10.23,-22.76,;7.96,-28.12,;6.71,-29.02,;9.43,-28.6,;10.57,-27.58,;10.25,-26.08,;12.03,-28.06,;12.35,-29.57,;11.19,-30.6,;9.73,-30.11,;8.58,-31.14,)| Show InChI InChI=1S/C17H12Cl2N6O/c1-24-9-13(15(23-24)14-11(18)4-2-5-12(14)19)22-17(26)10-8-21-25-7-3-6-20-16(10)25/h2-9H,1H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 in human TF1 cells assessed as reduction in STAT5 phosphorylation incubated for 30 mins in presence of human recombinant EPO |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399032

(CHEMBL2178256)Show InChI InChI=1S/C16H11ClN6O2/c17-10-3-1-2-9(6-10)14-12(8-20-25-14)21-16(24)11-7-19-23-5-4-13(18)22-15(11)23/h1-8H,(H2,18,22)(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399031

(CHEMBL2178257 | US8637526, 250)Show InChI InChI=1S/C16H11ClN6O2/c17-10-3-1-2-9(6-10)14-12(8-25-22-14)20-16(24)11-7-19-23-5-4-13(18)21-15(11)23/h1-8H,(H2,18,21)(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TRKC |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399033

(CHEMBL2178255)Show SMILES Nc1ccn2ncc(C(=O)Nc3c[nH]nc3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C16H12ClN7O/c17-10-3-1-2-9(6-10)14-12(8-19-23-14)21-16(25)11-7-20-24-5-4-13(18)22-15(11)24/h1-8H,(H2,18,22)(H,19,23)(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TRKA |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399038

(CHEMBL2178806)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-11-8-15(24(22-11)13-5-2-4-12(18)9-13)21-17(25)14-10-20-23-7-3-6-19-16(14)23/h2-10H,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Fyn |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399037

(CHEMBL2178807)Show SMILES Cc1cc(NC(=O)c2cnn3ccc(N)nc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-10-7-15(25(23-10)12-4-2-3-11(18)8-12)22-17(26)13-9-20-24-6-5-14(19)21-16(13)24/h2-9H,1H3,(H2,19,21)(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TRKB |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399030

(CHEMBL2178258)Show SMILES Cc1nc(c(NC(=O)c2cnn3ccc(N)nc23)s1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6OS/c1-9-21-14(10-3-2-4-11(18)7-10)17(26-9)23-16(25)12-8-20-24-6-5-13(19)22-15(12)24/h2-8H,1H3,(H2,19,22)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399034

(CHEMBL2178253)Show InChI InChI=1S/C16H11ClN6O/c17-11-4-1-3-10(7-11)14-13(9-19-22-14)21-16(24)12-8-20-23-6-2-5-18-15(12)23/h1-9H,(H,19,22)(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399011

(CHEMBL2178251)Show SMILES Cn1cc(c(NC(=O)c2cnn3cccnc23)n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(11-4-2-5-12(18)8-11)15(22-23)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399013

(CHEMBL2178248)Show InChI InChI=1S/C18H12ClN5O/c19-13-5-1-4-12(10-13)16-15(6-2-7-20-16)23-18(25)14-11-22-24-9-3-8-21-17(14)24/h1-11H,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399010

(CHEMBL2178252)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(15(22-23)11-4-2-5-12(18)8-11)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399036

(CHEMBL2178247)Show SMILES CNc1ccn2ncc(C(=O)Nc3cc(C)nn3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C18H16ClN7O/c1-11-8-16(26(24-11)13-5-3-4-12(19)9-13)23-18(27)14-10-21-25-7-6-15(20-2)22-17(14)25/h3-10H,1-2H3,(H,20,22)(H,23,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399037

(CHEMBL2178807)Show SMILES Cc1cc(NC(=O)c2cnn3ccc(N)nc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-10-7-15(25(23-10)12-4-2-3-11(18)8-12)22-17(26)13-9-20-24-6-5-14(19)21-16(13)24/h2-9H,1H3,(H2,19,21)(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399010

(CHEMBL2178252)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(15(22-23)11-4-2-5-12(18)8-11)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399009

(CHEMBL2178254)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-24-9-13(15(23-24)10-3-2-4-11(18)7-10)21-17(26)12-8-20-25-6-5-14(19)22-16(12)25/h2-9H,1H3,(H2,19,22)(H,21,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399011

(CHEMBL2178251)Show SMILES Cn1cc(c(NC(=O)c2cnn3cccnc23)n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(11-4-2-5-12(18)8-11)15(22-23)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399013

(CHEMBL2178248)Show InChI InChI=1S/C18H12ClN5O/c19-13-5-1-4-12(10-13)16-15(6-2-7-20-16)23-18(25)14-11-22-24-9-3-8-21-17(14)24/h1-11H,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399013

(CHEMBL2178248)Show InChI InChI=1S/C18H12ClN5O/c19-13-5-1-4-12(10-13)16-15(6-2-7-20-16)23-18(25)14-11-22-24-9-3-8-21-17(14)24/h1-11H,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399011

(CHEMBL2178251)Show SMILES Cn1cc(c(NC(=O)c2cnn3cccnc23)n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(11-4-2-5-12(18)8-11)15(22-23)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399012

(CHEMBL2178250)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)c(cn1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H14ClN5O/c1-12-8-17(15(10-22-12)13-4-2-5-14(20)9-13)24-19(26)16-11-23-25-7-3-6-21-18(16)25/h2-11H,1H3,(H,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399013

(CHEMBL2178248)Show InChI InChI=1S/C18H12ClN5O/c19-13-5-1-4-12(10-13)16-15(6-2-7-20-16)23-18(25)14-11-22-24-9-3-8-21-17(14)24/h1-11H,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399036

(CHEMBL2178247)Show SMILES CNc1ccn2ncc(C(=O)Nc3cc(C)nn3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C18H16ClN7O/c1-11-8-16(26(24-11)13-5-3-4-12(19)9-13)23-18(27)14-10-21-25-7-6-15(20-2)22-17(14)25/h3-10H,1-2H3,(H,20,22)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399010

(CHEMBL2178252)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(15(22-23)11-4-2-5-12(18)8-11)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399010

(CHEMBL2178252)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(15(22-23)11-4-2-5-12(18)8-11)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399013

(CHEMBL2178248)Show InChI InChI=1S/C18H12ClN5O/c19-13-5-1-4-12(10-13)16-15(6-2-7-20-16)23-18(25)14-11-22-24-9-3-8-21-17(14)24/h1-11H,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399012

(CHEMBL2178250)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)c(cn1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H14ClN5O/c1-12-8-17(15(10-22-12)13-4-2-5-14(20)9-13)24-19(26)16-11-23-25-7-3-6-21-18(16)25/h2-11H,1H3,(H,22,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399009

(CHEMBL2178254)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-24-9-13(15(23-24)10-3-2-4-11(18)7-10)21-17(26)12-8-20-25-6-5-14(19)22-16(12)25/h2-9H,1H3,(H2,19,22)(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399036

(CHEMBL2178247)Show SMILES CNc1ccn2ncc(C(=O)Nc3cc(C)nn3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C18H16ClN7O/c1-11-8-16(26(24-11)13-5-3-4-12(19)9-13)23-18(27)14-10-21-25-7-6-15(20-2)22-17(14)25/h3-10H,1-2H3,(H,20,22)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399036

(CHEMBL2178247)Show SMILES CNc1ccn2ncc(C(=O)Nc3cc(C)nn3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C18H16ClN7O/c1-11-8-16(26(24-11)13-5-3-4-12(19)9-13)23-18(27)14-10-21-25-7-6-15(20-2)22-17(14)25/h3-10H,1-2H3,(H,20,22)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399037

(CHEMBL2178807)Show SMILES Cc1cc(NC(=O)c2cnn3ccc(N)nc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-10-7-15(25(23-10)12-4-2-3-11(18)8-12)22-17(26)13-9-20-24-6-5-14(19)21-16(13)24/h2-9H,1H3,(H2,19,21)(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399012

(CHEMBL2178250)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)c(cn1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H14ClN5O/c1-12-8-17(15(10-22-12)13-4-2-5-14(20)9-13)24-19(26)16-11-23-25-7-3-6-21-18(16)25/h2-11H,1H3,(H,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399011

(CHEMBL2178251)Show SMILES Cn1cc(c(NC(=O)c2cnn3cccnc23)n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(11-4-2-5-12(18)8-11)15(22-23)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399012

(CHEMBL2178250)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)c(cn1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H14ClN5O/c1-12-8-17(15(10-22-12)13-4-2-5-14(20)9-13)24-19(26)16-11-23-25-7-3-6-21-18(16)25/h2-11H,1H3,(H,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399012

(CHEMBL2178250)Show SMILES Cc1cc(NC(=O)c2cnn3cccnc23)c(cn1)-c1cccc(Cl)c1 Show InChI InChI=1S/C19H14ClN5O/c1-12-8-17(15(10-22-12)13-4-2-5-14(20)9-13)24-19(26)16-11-23-25-7-3-6-21-18(16)25/h2-11H,1H3,(H,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399010

(CHEMBL2178252)Show SMILES Cn1cc(NC(=O)c2cnn3cccnc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(15(22-23)11-4-2-5-12(18)8-11)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399009

(CHEMBL2178254)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-24-9-13(15(23-24)10-3-2-4-11(18)7-10)21-17(26)12-8-20-25-6-5-14(19)22-16(12)25/h2-9H,1H3,(H2,19,22)(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399009

(CHEMBL2178254)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-24-9-13(15(23-24)10-3-2-4-11(18)7-10)21-17(26)12-8-20-25-6-5-14(19)22-16(12)25/h2-9H,1H3,(H2,19,22)(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50399011

(CHEMBL2178251)Show SMILES Cn1cc(c(NC(=O)c2cnn3cccnc23)n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H13ClN6O/c1-23-10-14(11-4-2-5-12(18)8-11)15(22-23)21-17(25)13-9-20-24-7-3-6-19-16(13)24/h2-10H,1H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50399036

(CHEMBL2178247)Show SMILES CNc1ccn2ncc(C(=O)Nc3cc(C)nn3-c3cccc(Cl)c3)c2n1 Show InChI InChI=1S/C18H16ClN7O/c1-11-8-16(26(24-11)13-5-3-4-12(19)9-13)23-18(27)14-10-21-25-7-6-15(20-2)22-17(14)25/h3-10H,1-2H3,(H,20,22)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399037

(CHEMBL2178807)Show SMILES Cc1cc(NC(=O)c2cnn3ccc(N)nc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-10-7-15(25(23-10)12-4-2-3-11(18)8-12)22-17(26)13-9-20-24-6-5-14(19)21-16(13)24/h2-9H,1H3,(H2,19,21)(H,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399037

(CHEMBL2178807)Show SMILES Cc1cc(NC(=O)c2cnn3ccc(N)nc23)n(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-10-7-15(25(23-10)12-4-2-3-11(18)8-12)22-17(26)13-9-20-24-6-5-14(19)21-16(13)24/h2-9H,1H3,(H2,19,21)(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399009

(CHEMBL2178254)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C17H14ClN7O/c1-24-9-13(15(23-24)10-3-2-4-11(18)7-10)21-17(26)12-8-20-25-6-5-14(19)22-16(12)25/h2-9H,1H3,(H2,19,22)(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50399015

(CHEMBL2178804)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N)nc23)c(n1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H13Cl2N7O/c1-25-8-13(15(24-25)10-6-9(18)2-3-12(10)19)22-17(27)11-7-21-26-5-4-14(20)23-16(11)26/h2-8H,1H3,(H2,20,23)(H,22,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of ROS |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data