

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402577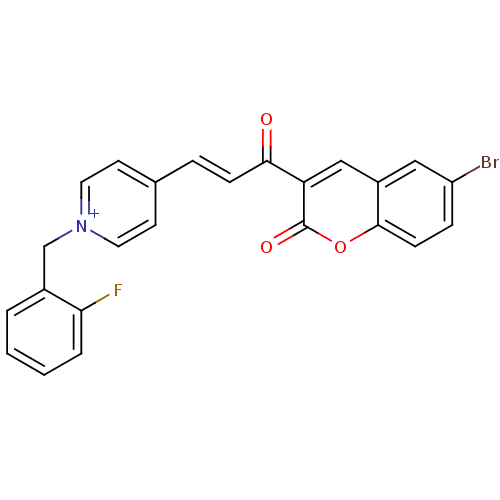 (CHEMBL2206129) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402583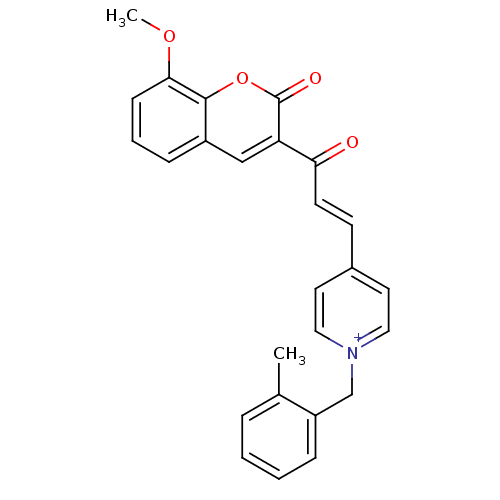 (CHEMBL2206123) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402576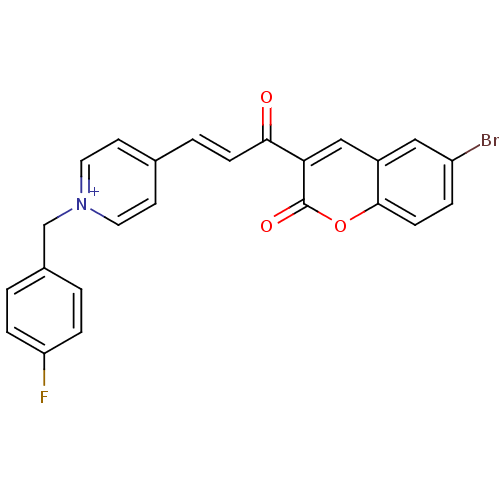 (CHEMBL2206130) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402587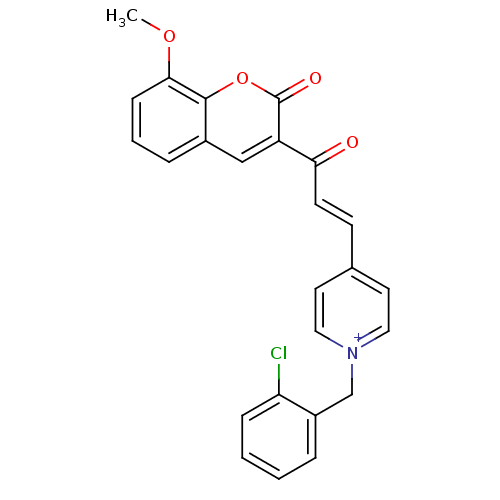 (CHEMBL2206119) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402590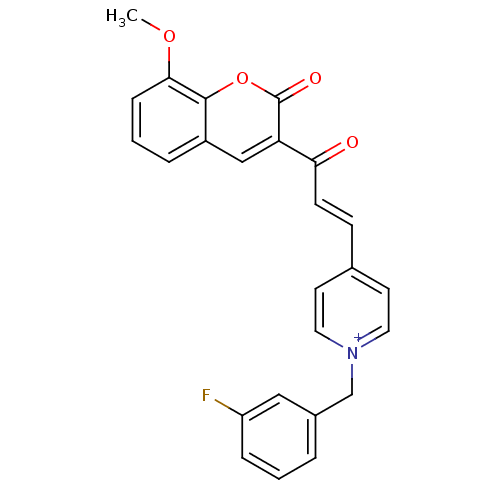 (CHEMBL2205576) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402591 (CHEMBL2205575) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402580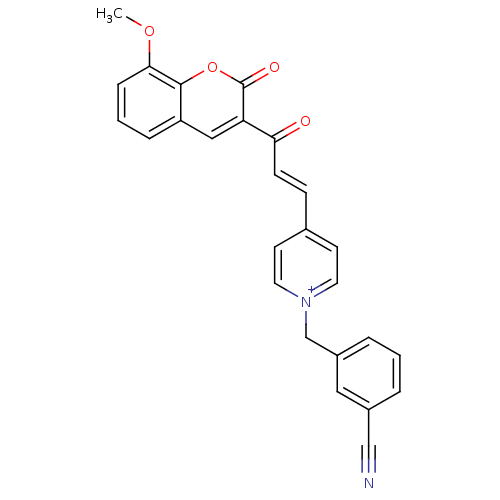 (CHEMBL2206126) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402586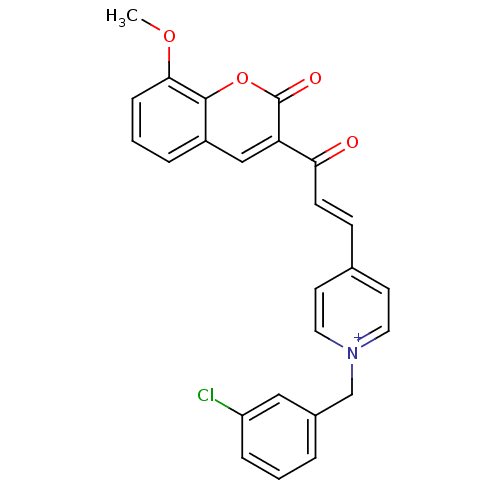 (CHEMBL2206120) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402585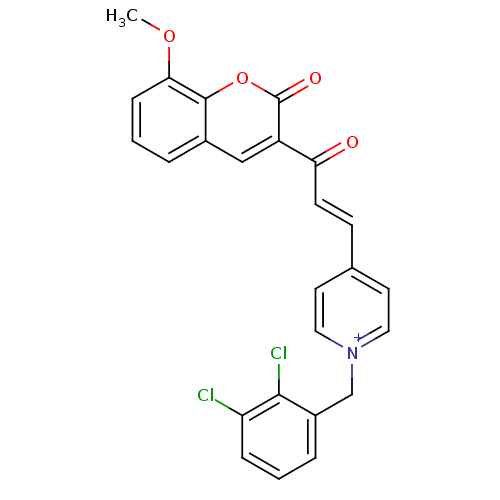 (CHEMBL2206121) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402587 (CHEMBL2206119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402591 (CHEMBL2205575) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402588 (CHEMBL2206118) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402583 (CHEMBL2206123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402584 (CHEMBL2206122) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402589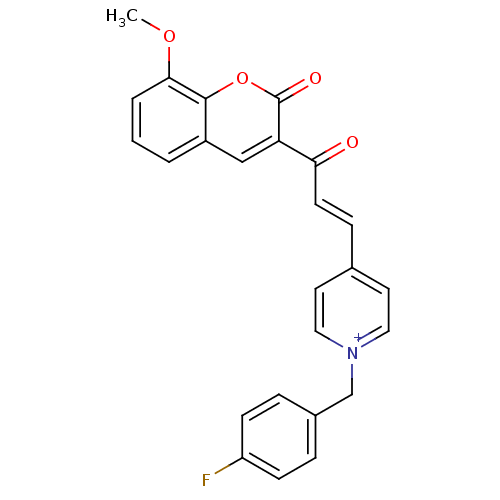 (CHEMBL2205577) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402577 (CHEMBL2206129) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402576 (CHEMBL2206130) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402593 (CHEMBL2205573) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402586 (CHEMBL2206120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402588 (CHEMBL2206118) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 685 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402592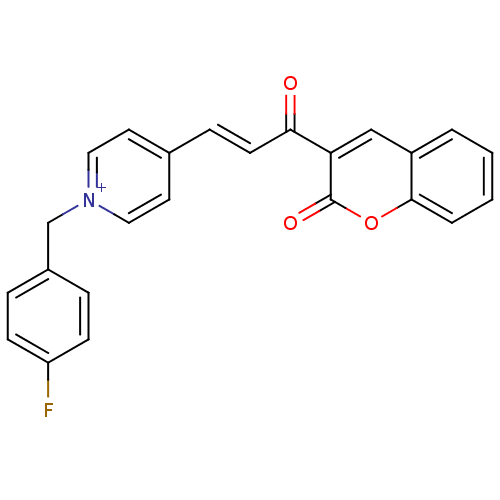 (CHEMBL2205574) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402582 (CHEMBL2206124) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402593 (CHEMBL2205573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 848 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402592 (CHEMBL2205574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402589 (CHEMBL2205577) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 931 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402578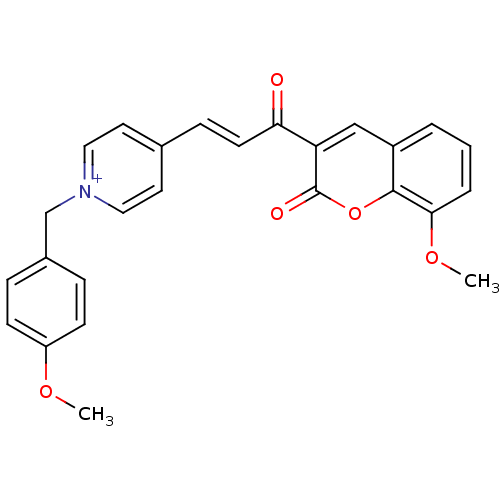 (CHEMBL2206128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 971 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402580 (CHEMBL2206126) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402578 (CHEMBL2206128) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402579 (CHEMBL2206127) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402590 (CHEMBL2205576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402581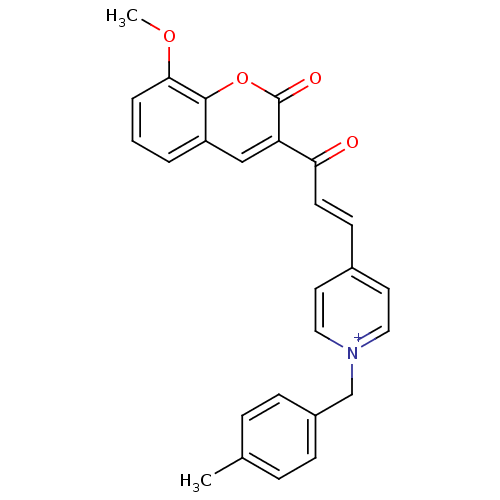 (CHEMBL2206125) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402584 (CHEMBL2206122) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402585 (CHEMBL2206121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402582 (CHEMBL2206124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402579 (CHEMBL2206127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50402581 (CHEMBL2206125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||