

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400044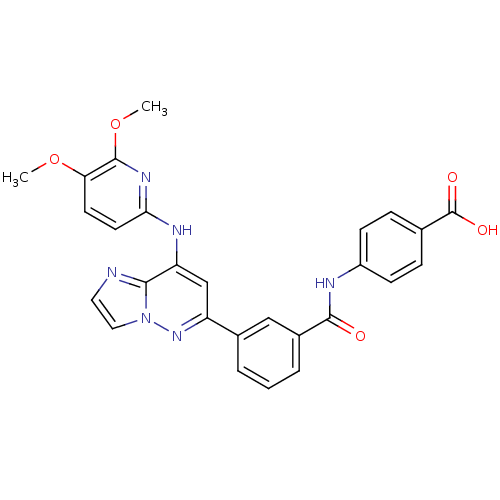 (CHEMBL2177726 | US9169259, I-54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400047 (BIIB-057 | CHEMBL2177736 | US9579320, Example 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400045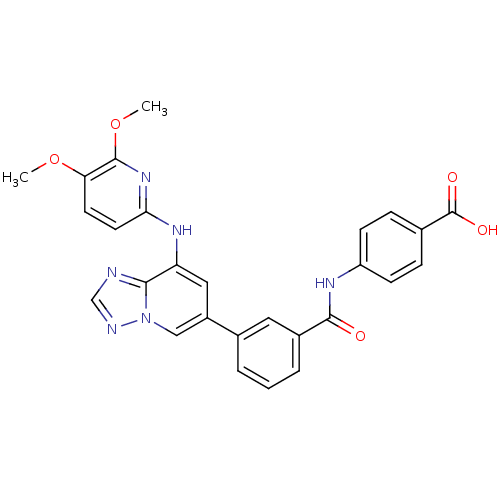 (CHEMBL2177725) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400046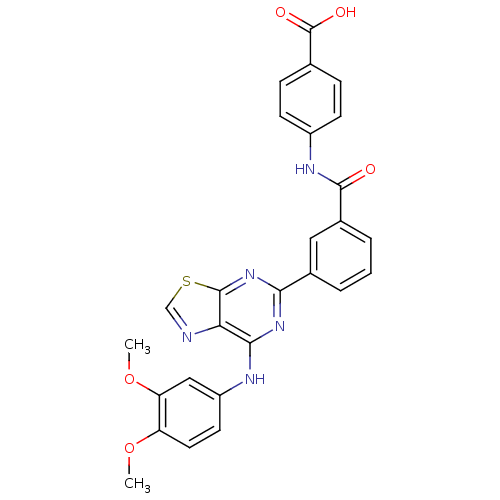 (CHEMBL2177737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400035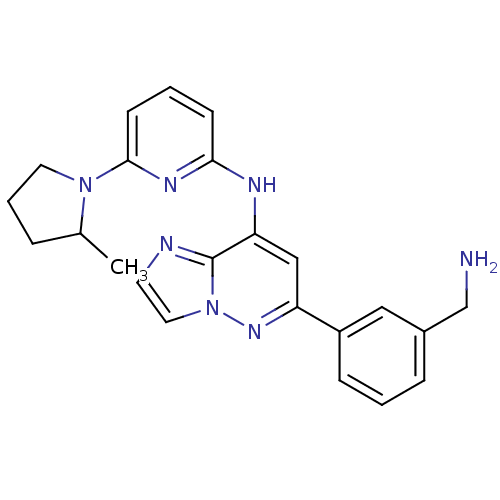 (CHEMBL2177735 | US9169259, I-84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400034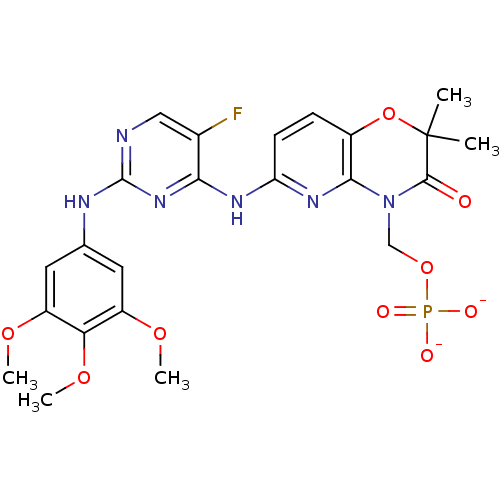 (FOSTAMATINIB DISODIUM | R788 SODIUM | R935788 SODI...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400036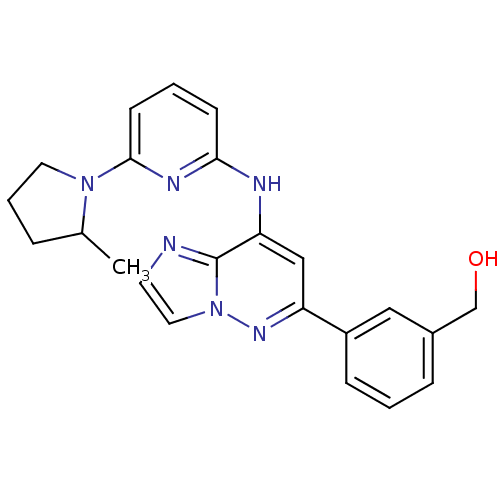 (CHEMBL2177734 | US9169259, I-68) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400040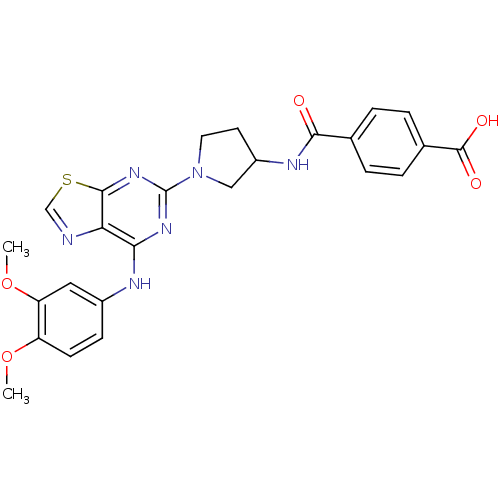 (CHEMBL2177730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400043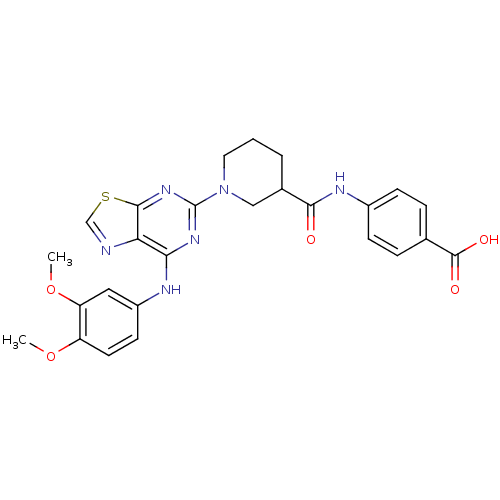 (CHEMBL2177727) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400042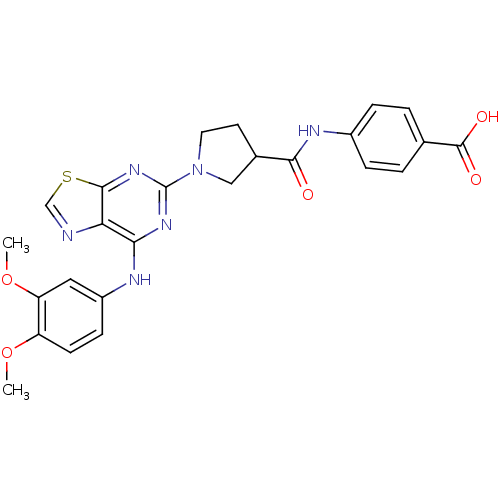 (CHEMBL2177728) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400037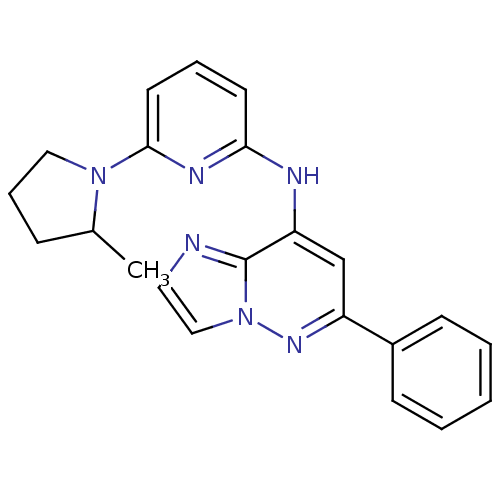 (CHEMBL2177733 | US9169259, I-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400038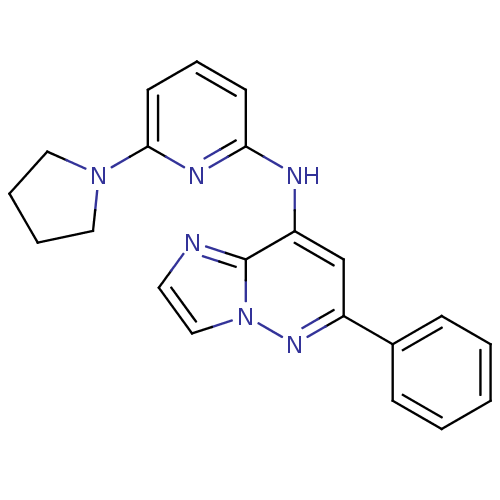 (CHEMBL2177732 | US9169259, I-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400039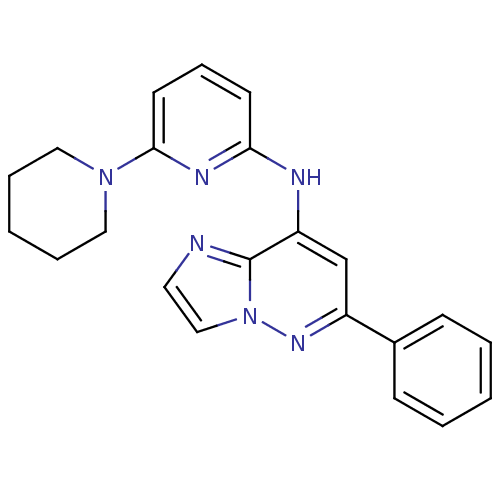 (CHEMBL2177731 | US9169259, I-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400041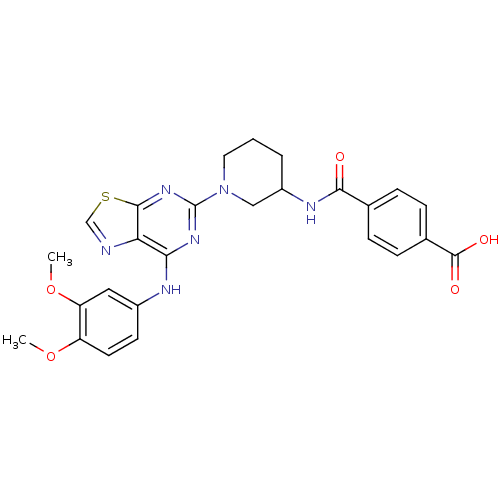 (CHEMBL2177729) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 423 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant spleen tyrosine kinase (360 to 635 amino acid residues) after 10 mins by scintillation counting analysis | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50400034 (FOSTAMATINIB DISODIUM | R788 SODIUM | R935788 SODI...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of vascular endothelial growth factor receptor 2 | J Med Chem 55: 10414-23 (2012) Article DOI: 10.1021/jm301367c BindingDB Entry DOI: 10.7270/Q2057H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||