 Found 38 hits of Enzyme Inhibition Constant Data
Found 38 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428742
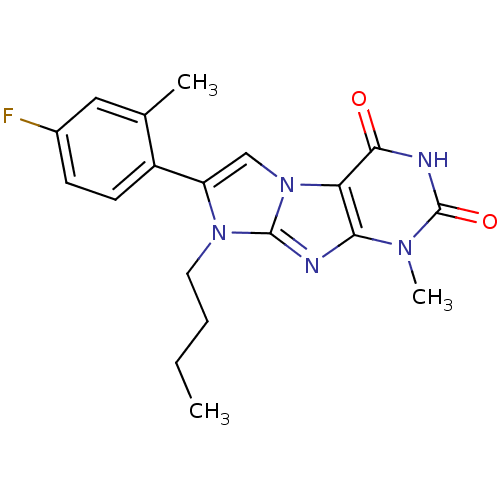
(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428744
(CHEMBL2333593)Show SMILES CCCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C20H23N5O2/c1-4-5-8-11-24-15(14-10-7-6-9-13(14)2)12-25-16-17(21-19(24)25)23(3)20(27)22-18(16)26/h6-7,9-10,12H,4-5,8,11H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
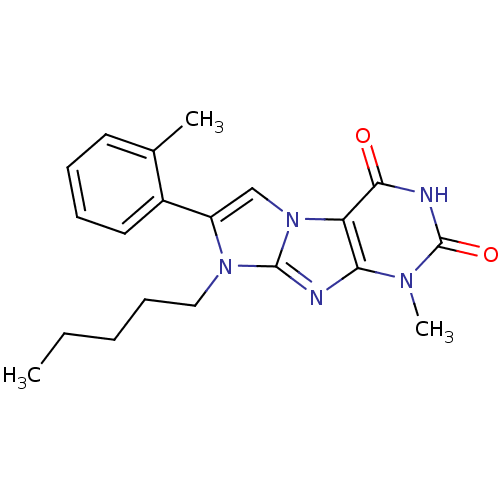
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428746
(CHEMBL2333597)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1Cl Show InChI InChI=1S/C18H18ClN5O2/c1-3-4-9-23-13(11-7-5-6-8-12(11)19)10-24-14-15(20-17(23)24)22(2)18(26)21-16(14)25/h5-8,10H,3-4,9H2,1-2H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428744

(CHEMBL2333593)Show SMILES CCCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C20H23N5O2/c1-4-5-8-11-24-15(14-10-7-6-9-13(14)2)12-25-16-17(21-19(24)25)23(3)20(27)22-18(16)26/h6-7,9-10,12H,4-5,8,11H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428746

(CHEMBL2333597)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1Cl Show InChI InChI=1S/C18H18ClN5O2/c1-3-4-9-23-13(11-7-5-6-8-12(11)19)10-24-14-15(20-17(23)24)22(2)18(26)21-16(14)25/h5-8,10H,3-4,9H2,1-2H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
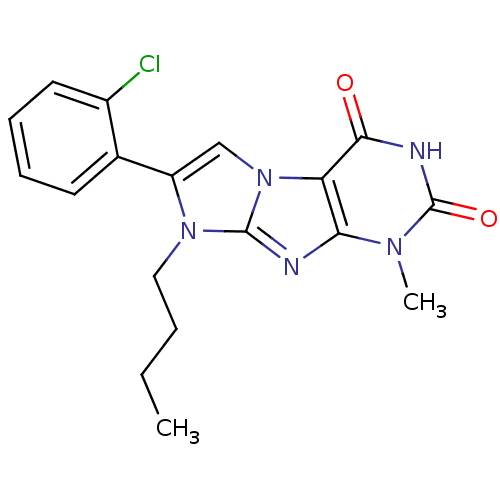
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428747
(CHEMBL2333600)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1Cl |(36.42,-49.25,;36.42,-47.71,;37.75,-46.94,;39.09,-47.71,;40.42,-46.94,;40.41,-45.39,;39.08,-44.63,;37.75,-45.4,;36.42,-44.63,;36.43,-43.09,;34.96,-42.61,;34.05,-43.85,;34.95,-45.1,;34.05,-46.35,;32.58,-45.87,;31.25,-46.63,;31.25,-48.17,;29.92,-45.87,;28.59,-46.64,;29.92,-44.33,;31.25,-43.55,;31.25,-42.01,;32.58,-44.33,;37.2,-41.76,;38.74,-41.76,;39.52,-40.43,;38.75,-39.1,;37.2,-39.1,;36.43,-40.43,;34.9,-40.43,)| Show InChI InChI=1S/C21H16ClN5O3/c1-25-18-17(19(28)24-21(25)29)26-11-15(12-7-3-4-8-13(12)22)27(20(26)23-18)14-9-5-6-10-16(14)30-2/h3-11H,1-2H3,(H,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50374167

(CHEMBL271967)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1 Show InChI InChI=1S/C18H19N5O2/c1-3-4-10-22-13(12-8-6-5-7-9-12)11-23-14-15(19-17(22)23)21(2)18(25)20-16(14)24/h5-9,11H,3-4,10H2,1-2H3,(H,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 538 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 578 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428745
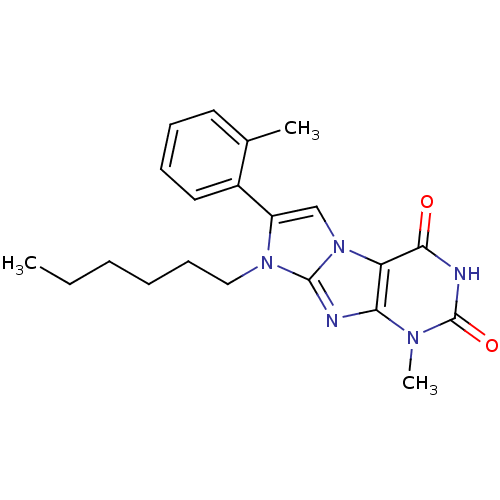
(CHEMBL2333594)Show SMILES CCCCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C21H25N5O2/c1-4-5-6-9-12-25-16(15-11-8-7-10-14(15)2)13-26-17-18(22-20(25)26)24(3)21(28)23-19(17)27/h7-8,10-11,13H,4-6,9,12H2,1-3H3,(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50374167

(CHEMBL271967)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1 Show InChI InChI=1S/C18H19N5O2/c1-3-4-10-22-13(12-8-6-5-7-9-12)11-23-14-15(19-17(22)23)21(2)18(25)20-16(14)24/h5-9,11H,3-4,10H2,1-2H3,(H,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
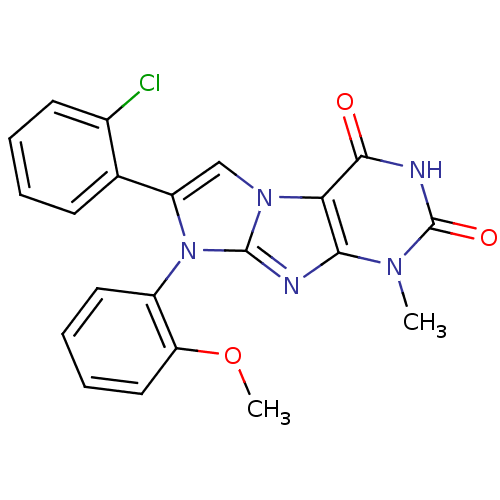
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428747

(CHEMBL2333600)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1Cl |(36.42,-49.25,;36.42,-47.71,;37.75,-46.94,;39.09,-47.71,;40.42,-46.94,;40.41,-45.39,;39.08,-44.63,;37.75,-45.4,;36.42,-44.63,;36.43,-43.09,;34.96,-42.61,;34.05,-43.85,;34.95,-45.1,;34.05,-46.35,;32.58,-45.87,;31.25,-46.63,;31.25,-48.17,;29.92,-45.87,;28.59,-46.64,;29.92,-44.33,;31.25,-43.55,;31.25,-42.01,;32.58,-44.33,;37.2,-41.76,;38.74,-41.76,;39.52,-40.43,;38.75,-39.1,;37.2,-39.1,;36.43,-40.43,;34.9,-40.43,)| Show InChI InChI=1S/C21H16ClN5O3/c1-25-18-17(19(28)24-21(25)29)26-11-15(12-7-3-4-8-13(12)22)27(20(26)23-18)14-9-5-6-10-16(14)30-2/h3-11H,1-2H3,(H,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299226

(8-(2-methoxyphenyl)-1-methyl-7-phenyl-1H-imidazo[1...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1 |(16.51,-2.42,;16.51,-.88,;17.85,-.11,;19.17,-.88,;20.5,-.11,;20.5,1.43,;19.17,2.19,;17.85,1.42,;16.52,2.19,;16.53,3.73,;15.06,4.22,;14.15,2.97,;15.05,1.72,;14.15,.48,;12.68,.95,;11.35,.19,;11.35,-1.35,;10.03,.96,;8.69,.18,;10.03,2.5,;11.35,3.27,;11.35,4.81,;12.68,2.5,;17.86,4.51,;19.2,3.74,;20.52,4.52,;20.52,6.06,;19.17,6.82,;17.84,6.04,)| Show InChI InChI=1S/C21H17N5O3/c1-24-18-17(19(27)23-21(24)28)25-12-15(13-8-4-3-5-9-13)26(20(25)22-18)14-10-6-7-11-16(14)29-2/h3-12H,1-2H3,(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299226

(8-(2-methoxyphenyl)-1-methyl-7-phenyl-1H-imidazo[1...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1 |(16.51,-2.42,;16.51,-.88,;17.85,-.11,;19.17,-.88,;20.5,-.11,;20.5,1.43,;19.17,2.19,;17.85,1.42,;16.52,2.19,;16.53,3.73,;15.06,4.22,;14.15,2.97,;15.05,1.72,;14.15,.48,;12.68,.95,;11.35,.19,;11.35,-1.35,;10.03,.96,;8.69,.18,;10.03,2.5,;11.35,3.27,;11.35,4.81,;12.68,2.5,;17.86,4.51,;19.2,3.74,;20.52,4.52,;20.52,6.06,;19.17,6.82,;17.84,6.04,)| Show InChI InChI=1S/C21H17N5O3/c1-24-18-17(19(27)23-21(24)28)25-12-15(13-8-4-3-5-9-13)26(20(25)22-18)14-10-6-7-11-16(14)29-2/h3-12H,1-2H3,(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data