 Found 49 hits of Enzyme Inhibition Constant Data
Found 49 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426788
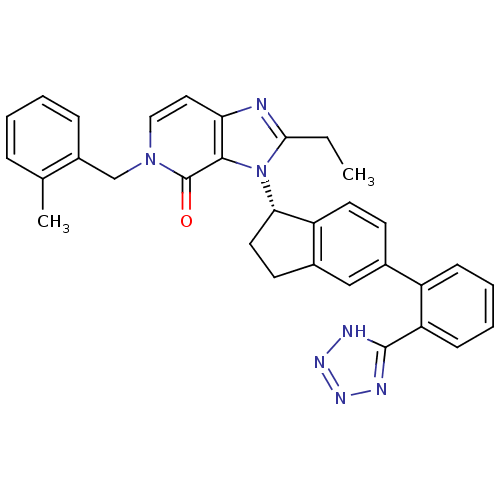
(CHEMBL2322444)Show SMILES CCc1nc2ccn(Cc3ccccc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7O/c1-3-29-33-27-16-17-38(19-23-9-5-4-8-20(23)2)32(40)30(27)39(29)28-15-13-22-18-21(12-14-25(22)28)24-10-6-7-11-26(24)31-34-36-37-35-31/h4-12,14,16-18,28H,3,13,15,19H2,1-2H3,(H,34,35,36,37)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426777
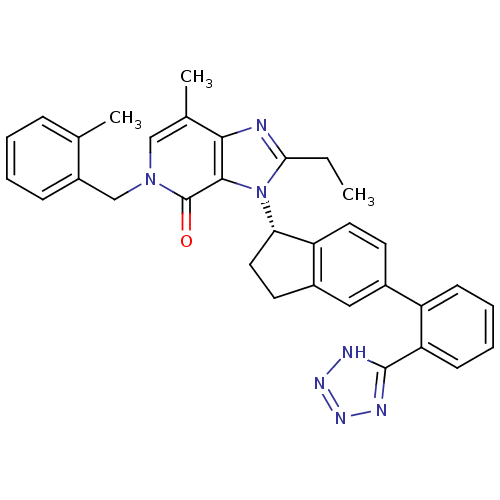
(CHEMBL2322175)Show SMILES CCc1nc2c(C)cn(Cc3ccccc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H31N7O/c1-4-29-34-30-21(3)18-39(19-24-10-6-5-9-20(24)2)33(41)31(30)40(29)28-16-14-23-17-22(13-15-26(23)28)25-11-7-8-12-27(25)32-35-37-38-36-32/h5-13,15,17-18,28H,4,14,16,19H2,1-3H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426776
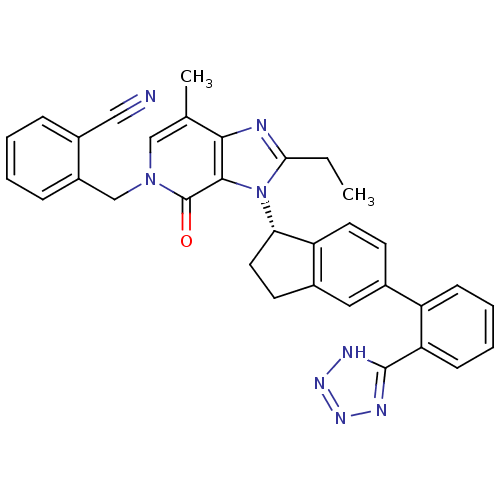
(CHEMBL2322176)Show SMILES CCc1nc2c(C)cn(Cc3ccccc3C#N)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H28N8O/c1-3-29-35-30-20(2)18-40(19-24-9-5-4-8-23(24)17-34)33(42)31(30)41(29)28-15-13-22-16-21(12-14-26(22)28)25-10-6-7-11-27(25)32-36-38-39-37-32/h4-12,14,16,18,28H,3,13,15,19H2,1-2H3,(H,36,37,38,39)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426774
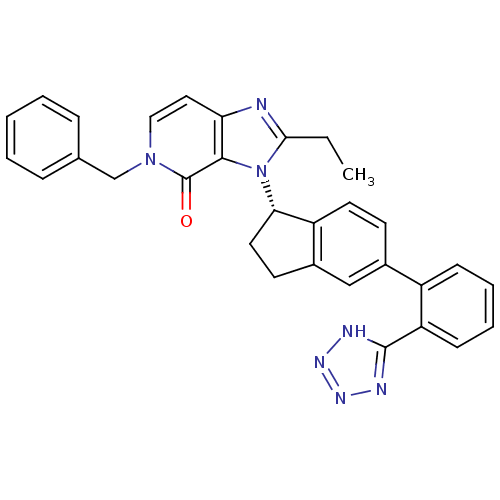
(CHEMBL2322437)Show SMILES CCc1nc2ccn(Cc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H27N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h3-12,14,16-18,27H,2,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426787
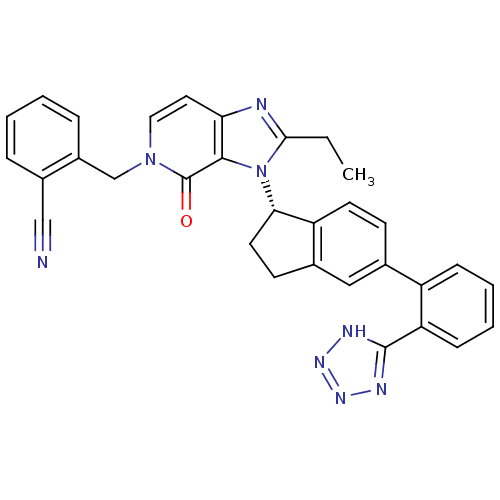
(CHEMBL2322445)Show SMILES CCc1nc2ccn(Cc3ccccc3C#N)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26N8O/c1-2-29-34-27-15-16-39(19-23-8-4-3-7-22(23)18-33)32(41)30(27)40(29)28-14-12-21-17-20(11-13-25(21)28)24-9-5-6-10-26(24)31-35-37-38-36-31/h3-11,13,15-17,28H,2,12,14,19H2,1H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426770
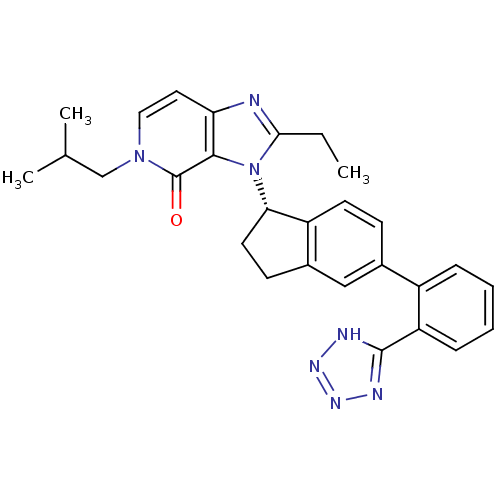
(CHEMBL2322441)Show SMILES CCc1nc2ccn(CC(C)C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H29N7O/c1-4-25-29-23-13-14-34(16-17(2)3)28(36)26(23)35(25)24-12-10-19-15-18(9-11-21(19)24)20-7-5-6-8-22(20)27-30-32-33-31-27/h5-9,11,13-15,17,24H,4,10,12,16H2,1-3H3,(H,30,31,32,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426772

(CHEMBL2322439)Show SMILES CCc1nc2ccn(CC(=O)OCc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H29N7O3/c1-2-29-34-27-16-17-39(19-30(41)43-20-21-8-4-3-5-9-21)33(42)31(27)40(29)28-15-13-23-18-22(12-14-25(23)28)24-10-6-7-11-26(24)32-35-37-38-36-32/h3-12,14,16-18,28H,2,13,15,19-20H2,1H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426773
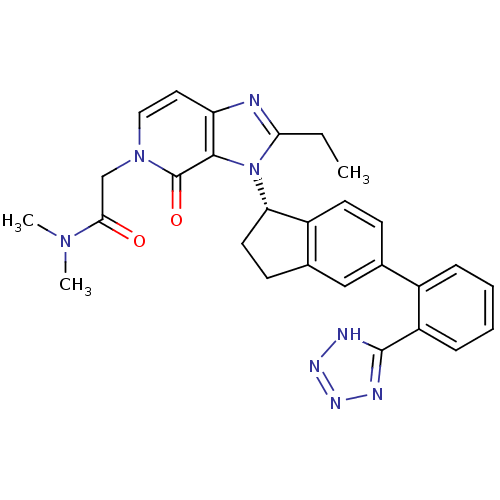
(CHEMBL2322438)Show SMILES CCc1nc2ccn(CC(=O)N(C)C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H28N8O2/c1-4-24-29-22-13-14-35(16-25(37)34(2)3)28(38)26(22)36(24)23-12-10-18-15-17(9-11-20(18)23)19-7-5-6-8-21(19)27-30-32-33-31-27/h5-9,11,13-15,23H,4,10,12,16H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426785
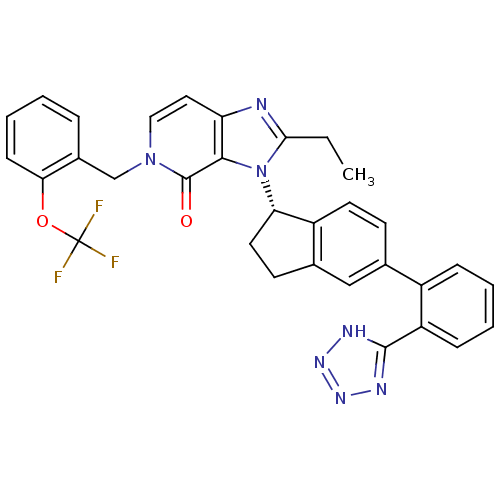
(CHEMBL2322447)Show SMILES CCc1nc2ccn(Cc3ccccc3OC(F)(F)F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26F3N7O2/c1-2-28-36-25-15-16-41(18-21-7-3-6-10-27(21)44-32(33,34)35)31(43)29(25)42(28)26-14-12-20-17-19(11-13-23(20)26)22-8-4-5-9-24(22)30-37-39-40-38-30/h3-11,13,15-17,26H,2,12,14,18H2,1H3,(H,37,38,39,40)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426783

(CHEMBL2322449)Show SMILES CCc1nc2ccn(Cc3cccc(C)c3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7O/c1-3-29-33-27-15-16-38(19-21-8-6-7-20(2)17-21)32(40)30(27)39(29)28-14-12-23-18-22(11-13-25(23)28)24-9-4-5-10-26(24)31-34-36-37-35-31/h4-11,13,15-18,28H,3,12,14,19H2,1-2H3,(H,34,35,36,37)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426789
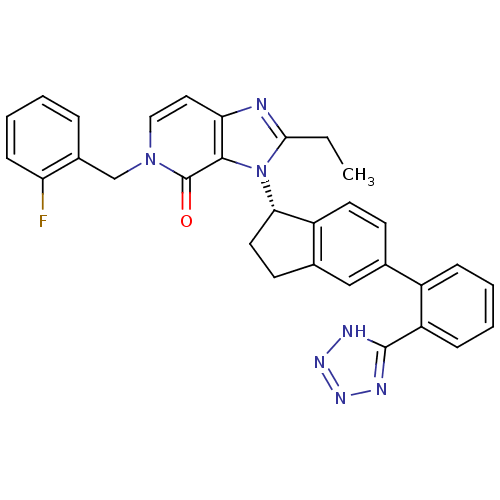
(CHEMBL2322443)Show SMILES CCc1nc2ccn(Cc3ccccc3F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H26FN7O/c1-2-28-33-26-15-16-38(18-21-7-3-6-10-25(21)32)31(40)29(26)39(28)27-14-12-20-17-19(11-13-23(20)27)22-8-4-5-9-24(22)30-34-36-37-35-30/h3-11,13,15-17,27H,2,12,14,18H2,1H3,(H,34,35,36,37)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426790
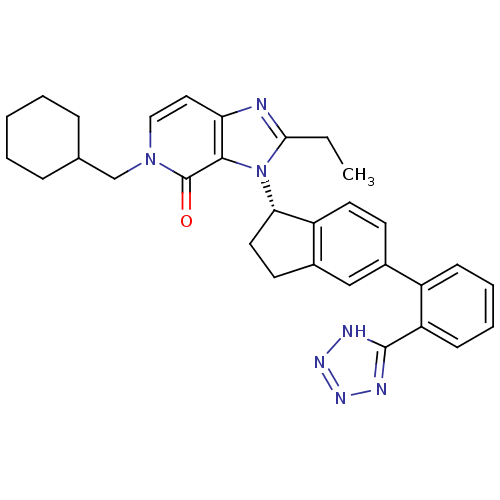
(CHEMBL2322442)Show SMILES CCc1nc2ccn(CC3CCCCC3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H33N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h6-7,10-12,14,16-18,20,27H,2-5,8-9,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426780
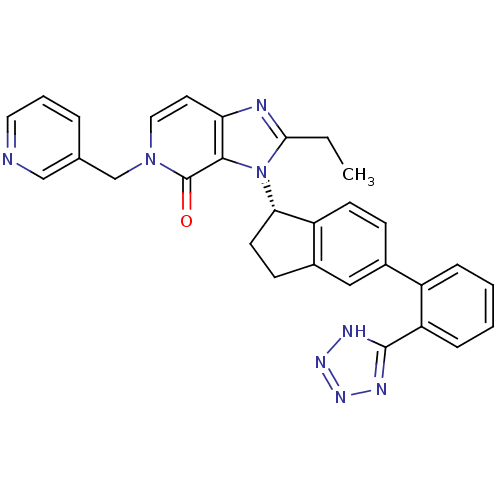
(CHEMBL2322172)Show SMILES CCc1nc2ccn(Cc3cccnc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H26N8O/c1-2-27-32-25-13-15-37(18-19-6-5-14-31-17-19)30(39)28(25)38(27)26-12-10-21-16-20(9-11-23(21)26)22-7-3-4-8-24(22)29-33-35-36-34-29/h3-9,11,13-17,26H,2,10,12,18H2,1H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426778
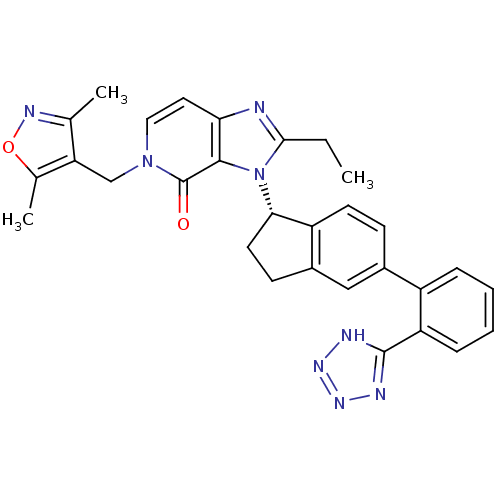
(CHEMBL2322174)Show SMILES CCc1nc2ccn(Cc3c(C)noc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H28N8O2/c1-4-27-31-25-13-14-37(16-24-17(2)34-40-18(24)3)30(39)28(25)38(27)26-12-10-20-15-19(9-11-22(20)26)21-7-5-6-8-23(21)29-32-35-36-33-29/h5-9,11,13-15,26H,4,10,12,16H2,1-3H3,(H,32,33,35,36)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426781
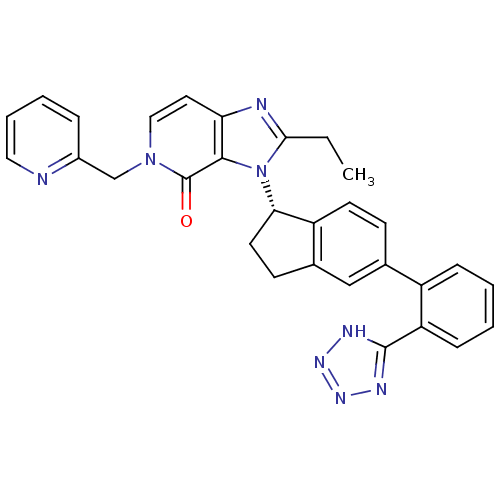
(CHEMBL2322451)Show SMILES CCc1nc2ccn(Cc3ccccn3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H26N8O/c1-2-27-32-25-14-16-37(18-21-7-5-6-15-31-21)30(39)28(25)38(27)26-13-11-20-17-19(10-12-23(20)26)22-8-3-4-9-24(22)29-33-35-36-34-29/h3-10,12,14-17,26H,2,11,13,18H2,1H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426784

(CHEMBL2322448)Show SMILES CCc1nc2ccn(Cc3cccc(F)c3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H26FN7O/c1-2-28-33-26-14-15-38(18-19-6-5-7-22(32)16-19)31(40)29(26)39(28)27-13-11-21-17-20(10-12-24(21)27)23-8-3-4-9-25(23)30-34-36-37-35-30/h3-10,12,14-17,27H,2,11,13,18H2,1H3,(H,34,35,36,37)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426782
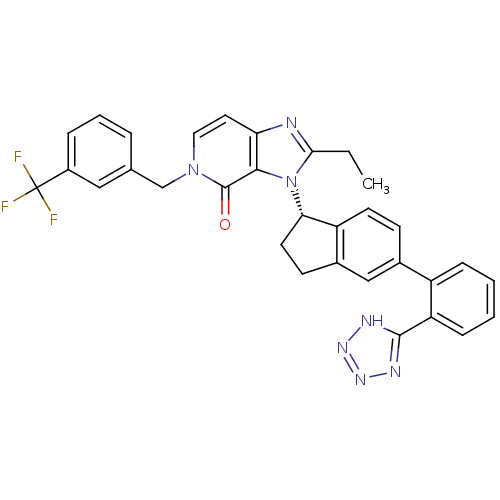
(CHEMBL2322450)Show SMILES CCc1nc2ccn(Cc3cccc(c3)C(F)(F)F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26F3N7O/c1-2-28-36-26-14-15-41(18-19-6-5-7-22(16-19)32(33,34)35)31(43)29(26)42(28)27-13-11-21-17-20(10-12-24(21)27)23-8-3-4-9-25(23)30-37-39-40-38-30/h3-10,12,14-17,27H,2,11,13,18H2,1H3,(H,37,38,39,40)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426786

(CHEMBL2322446)Show SMILES CCc1nc2ccn(Cc3ccccc3C(F)(F)F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26F3N7O/c1-2-28-36-26-15-16-41(18-21-7-3-6-10-25(21)32(33,34)35)31(43)29(26)42(28)27-14-12-20-17-19(11-13-23(20)27)22-8-4-5-9-24(22)30-37-39-40-38-30/h3-11,13,15-17,27H,2,12,14,18H2,1H3,(H,37,38,39,40)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426779
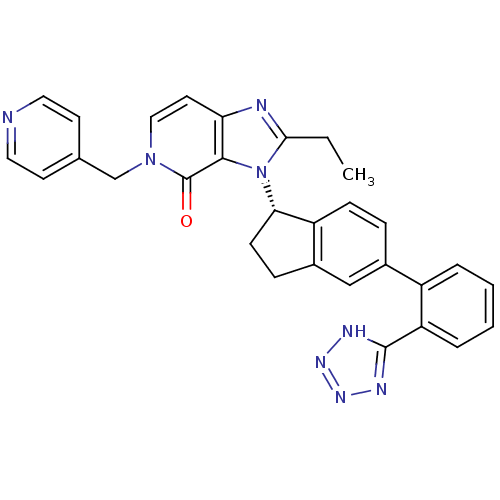
(CHEMBL2322173)Show SMILES CCc1nc2ccn(Cc3ccncc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H26N8O/c1-2-27-32-25-13-16-37(18-19-11-14-31-15-12-19)30(39)28(25)38(27)26-10-8-21-17-20(7-9-23(21)26)22-5-3-4-6-24(22)29-33-35-36-34-29/h3-7,9,11-17,26H,2,8,10,18H2,1H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426771
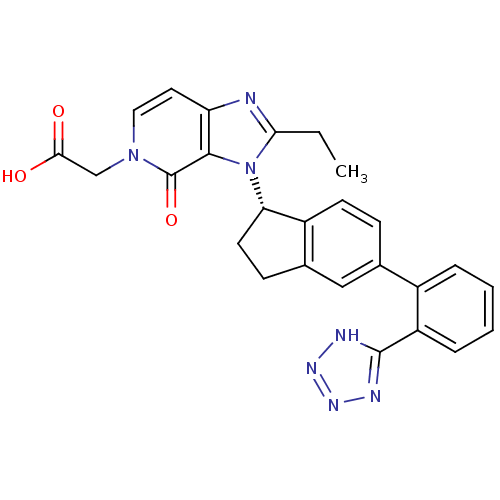
(CHEMBL2322440)Show SMILES CCc1nc2ccn(CC(O)=O)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H23N7O3/c1-2-22-27-20-11-12-32(14-23(34)35)26(36)24(20)33(22)21-10-8-16-13-15(7-9-18(16)21)17-5-3-4-6-19(17)25-28-30-31-29-25/h3-7,9,11-13,21H,2,8,10,14H2,1H3,(H,34,35)(H,28,29,30,31)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426775
(CHEMBL2322177)Show SMILES CCc1nc2cc[nH]c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C24H21N7O/c1-2-21-26-19-11-12-25-24(32)22(19)31(21)20-10-8-15-13-14(7-9-17(15)20)16-5-3-4-6-18(16)23-27-29-30-28-23/h3-7,9,11-13,20H,2,8,10H2,1H3,(H,25,32)(H,27,28,29,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 593 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426784

(CHEMBL2322448)Show SMILES CCc1nc2ccn(Cc3cccc(F)c3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H26FN7O/c1-2-28-33-26-14-15-38(18-19-6-5-7-22(32)16-19)31(40)29(26)39(28)27-13-11-21-17-20(10-12-24(21)27)23-8-3-4-9-25(23)30-34-36-37-35-30/h3-10,12,14-17,27H,2,11,13,18H2,1H3,(H,34,35,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 159 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426772

(CHEMBL2322439)Show SMILES CCc1nc2ccn(CC(=O)OCc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H29N7O3/c1-2-29-34-27-16-17-39(19-30(41)43-20-21-8-4-3-5-9-21)33(42)31(27)40(29)28-15-13-23-18-22(12-14-25(23)28)24-10-6-7-11-26(24)32-35-37-38-36-32/h3-12,14,16-18,28H,2,13,15,19-20H2,1H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426783

(CHEMBL2322449)Show SMILES CCc1nc2ccn(Cc3cccc(C)c3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7O/c1-3-29-33-27-15-16-38(19-21-8-6-7-20(2)17-21)32(40)30(27)39(29)28-14-12-23-18-22(11-13-25(23)28)24-9-4-5-10-26(24)31-34-36-37-35-31/h4-11,13,15-18,28H,3,12,14,19H2,1-2H3,(H,34,35,36,37)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 108 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426774

(CHEMBL2322437)Show SMILES CCc1nc2ccn(Cc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H27N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h3-12,14,16-18,27H,2,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 292 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426787

(CHEMBL2322445)Show SMILES CCc1nc2ccn(Cc3ccccc3C#N)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26N8O/c1-2-29-34-27-15-16-39(19-23-8-4-3-7-22(23)18-33)32(41)30(27)40(29)28-14-12-21-17-20(11-13-25(21)28)24-9-5-6-10-26(24)31-35-37-38-36-31/h3-11,13,15-17,28H,2,12,14,19H2,1H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 685 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426785

(CHEMBL2322447)Show SMILES CCc1nc2ccn(Cc3ccccc3OC(F)(F)F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26F3N7O2/c1-2-28-36-25-15-16-41(18-21-7-3-6-10-27(21)44-32(33,34)35)31(43)29(25)42(28)26-14-12-20-17-19(11-13-23(20)26)22-8-4-5-9-24(22)30-37-39-40-38-30/h3-11,13,15-17,26H,2,12,14,18H2,1H3,(H,37,38,39,40)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426779

(CHEMBL2322173)Show SMILES CCc1nc2ccn(Cc3ccncc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H26N8O/c1-2-27-32-25-13-16-37(18-19-11-14-31-15-12-19)30(39)28(25)38(27)26-10-8-21-17-20(7-9-23(21)26)22-5-3-4-6-24(22)29-33-35-36-34-29/h3-7,9,11-17,26H,2,8,10,18H2,1H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 212 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426771

(CHEMBL2322440)Show SMILES CCc1nc2ccn(CC(O)=O)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H23N7O3/c1-2-22-27-20-11-12-32(14-23(34)35)26(36)24(20)33(22)21-10-8-16-13-15(7-9-18(16)21)17-5-3-4-6-19(17)25-28-30-31-29-25/h3-7,9,11-13,21H,2,8,10,14H2,1H3,(H,34,35)(H,28,29,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426777

(CHEMBL2322175)Show SMILES CCc1nc2c(C)cn(Cc3ccccc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H31N7O/c1-4-29-34-30-21(3)18-39(19-24-10-6-5-9-20(24)2)33(41)31(30)40(29)28-16-14-23-17-22(13-15-26(23)28)25-11-7-8-12-27(25)32-35-37-38-36-32/h5-13,15,17-18,28H,4,14,16,19H2,1-3H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049240
((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,22H,2,9-11H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426786

(CHEMBL2322446)Show SMILES CCc1nc2ccn(Cc3ccccc3C(F)(F)F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26F3N7O/c1-2-28-36-26-15-16-41(18-21-7-3-6-10-25(21)32(33,34)35)31(43)29(26)42(28)27-14-12-20-17-19(11-13-23(20)27)22-8-4-5-9-24(22)30-37-39-40-38-30/h3-11,13,15-17,27H,2,12,14,18H2,1H3,(H,37,38,39,40)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 187 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426781

(CHEMBL2322451)Show SMILES CCc1nc2ccn(Cc3ccccn3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H26N8O/c1-2-27-32-25-14-16-37(18-21-7-5-6-15-31-21)30(39)28(25)38(27)26-13-11-20-17-19(10-12-23(20)26)22-8-3-4-9-24(22)29-33-35-36-34-29/h3-10,12,14-17,26H,2,11,13,18H2,1H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426788

(CHEMBL2322444)Show SMILES CCc1nc2ccn(Cc3ccccc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7O/c1-3-29-33-27-16-17-38(19-23-9-5-4-8-20(23)2)32(40)30(27)39(29)28-15-13-22-18-21(12-14-25(22)28)24-10-6-7-11-26(24)31-34-36-37-35-31/h4-12,14,16-18,28H,3,13,15,19H2,1-2H3,(H,34,35,36,37)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50426774

(CHEMBL2322437)Show SMILES CCc1nc2ccn(Cc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H27N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h3-12,14,16-18,27H,2,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha (unknown origin) |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426789

(CHEMBL2322443)Show SMILES CCc1nc2ccn(Cc3ccccc3F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H26FN7O/c1-2-28-33-26-15-16-38(18-21-7-3-6-10-25(21)32)31(40)29(26)39(28)27-14-12-20-17-19(11-13-23(20)27)22-8-4-5-9-24(22)30-34-36-37-35-30/h3-11,13,15-17,27H,2,12,14,18H2,1H3,(H,34,35,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426780

(CHEMBL2322172)Show SMILES CCc1nc2ccn(Cc3cccnc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H26N8O/c1-2-27-32-25-13-15-37(18-19-6-5-14-31-17-19)30(39)28(25)38(27)26-12-10-21-16-20(9-11-23(21)26)22-7-3-4-8-24(22)29-33-35-36-34-29/h3-9,11,13-17,26H,2,10,12,18H2,1H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426778

(CHEMBL2322174)Show SMILES CCc1nc2ccn(Cc3c(C)noc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H28N8O2/c1-4-27-31-25-13-14-37(16-24-17(2)34-40-18(24)3)30(39)28(25)38(27)26-12-10-20-15-19(9-11-22(20)26)21-7-5-6-8-23(21)29-32-35-36-33-29/h5-9,11,13-15,26H,4,10,12,16H2,1-3H3,(H,32,33,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426775

(CHEMBL2322177)Show SMILES CCc1nc2cc[nH]c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C24H21N7O/c1-2-21-26-19-11-12-25-24(32)22(19)31(21)20-10-8-15-13-14(7-9-17(15)20)16-5-3-4-6-18(16)23-27-29-30-28-23/h3-7,9,11-13,20H,2,8,10H2,1H3,(H,25,32)(H,27,28,29,30)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426773

(CHEMBL2322438)Show SMILES CCc1nc2ccn(CC(=O)N(C)C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H28N8O2/c1-4-24-29-22-13-14-35(16-25(37)34(2)3)28(38)26(22)36(24)23-12-10-18-15-17(9-11-20(18)23)19-7-5-6-8-21(19)27-30-32-33-31-27/h5-9,11,13-15,23H,4,10,12,16H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426770

(CHEMBL2322441)Show SMILES CCc1nc2ccn(CC(C)C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H29N7O/c1-4-25-29-23-13-14-34(16-17(2)3)28(36)26(23)35(25)24-12-10-19-15-18(9-11-21(19)24)20-7-5-6-8-22(20)27-30-32-33-31-27/h5-9,11,13-15,17,24H,4,10,12,16H2,1-3H3,(H,30,31,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426790

(CHEMBL2322442)Show SMILES CCc1nc2ccn(CC3CCCCC3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H33N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h6-7,10-12,14,16-18,20,27H,2-5,8-9,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 942 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426776

(CHEMBL2322176)Show SMILES CCc1nc2c(C)cn(Cc3ccccc3C#N)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H28N8O/c1-3-29-35-30-20(2)18-40(19-24-9-5-4-8-23(24)17-34)33(42)31(30)41(29)28-15-13-22-16-21(12-14-26(22)28)25-10-6-7-11-27(25)32-36-38-39-37-32/h4-12,14,16,18,28H,3,13,15,19H2,1-2H3,(H,36,37,38,39)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 295 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50426774

(CHEMBL2322437)Show SMILES CCc1nc2ccn(Cc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H27N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h3-12,14,16-18,27H,2,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARbeta (unknown origin) |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50426782

(CHEMBL2322450)Show SMILES CCc1nc2ccn(Cc3cccc(c3)C(F)(F)F)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26F3N7O/c1-2-28-36-26-14-15-41(18-19-6-5-7-22(16-19)32(33,34)35)31(43)29(26)42(28)27-13-11-21-17-20(10-12-24(21)27)23-8-3-4-9-25(23)30-37-39-40-38-30/h3-10,12,14-17,27H,2,11,13,18H2,1H3,(H,37,38,39,40)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 449 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data