

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246510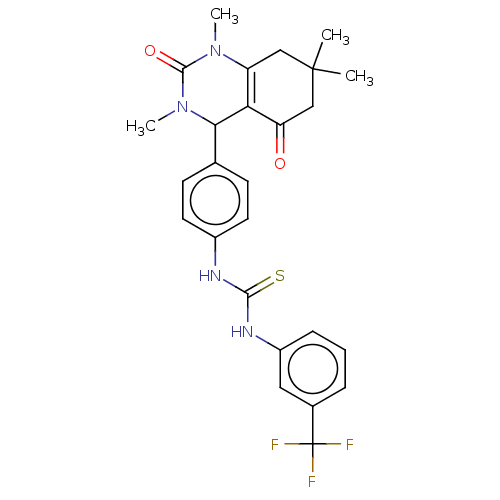 (1-(4-(1,3,7,7-Tetramethyl-5-oxo-1,2,3,4,5,6,7,8-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246507 (1-(4-Fluorophenyl)-3-(4-(1,3,7,7-tetramethyl-5-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246508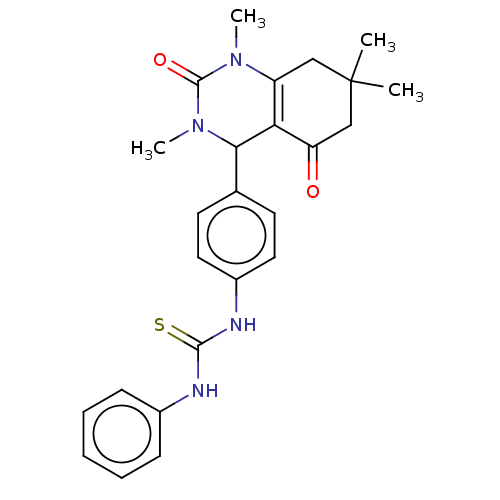 (1-Phenyl-3-(4-(1,3,7,7-tetramethyl-5-oxo-1,2,3,4,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246513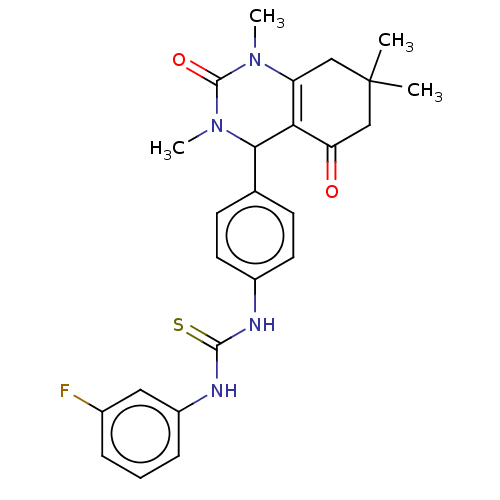 (1-(3-Fluorophenyl)-3-(4-(1,3,7,7-tetramethyl-5-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246510 (1-(4-(1,3,7,7-Tetramethyl-5-oxo-1,2,3,4,5,6,7,8-oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246513 (1-(3-Fluorophenyl)-3-(4-(1,3,7,7-tetramethyl-5-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246512 (1-(2,4-Dichlorophenyl)-3-(4-(1,3,7,7-tetramethyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246505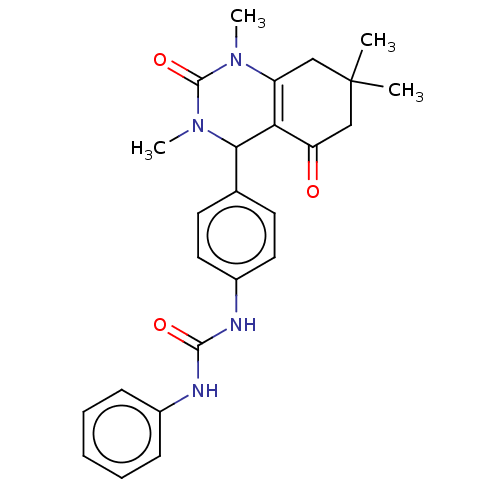 (1-Phenyl-3-(4-(1,3,7,7-tetramethyl-5-oxo-1,2,3,4,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246508 (1-Phenyl-3-(4-(1,3,7,7-tetramethyl-5-oxo-1,2,3,4,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246507 (1-(4-Fluorophenyl)-3-(4-(1,3,7,7-tetramethyl-5-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246512 (1-(2,4-Dichlorophenyl)-3-(4-(1,3,7,7-tetramethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246505 (1-Phenyl-3-(4-(1,3,7,7-tetramethyl-5-oxo-1,2,3,4,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246506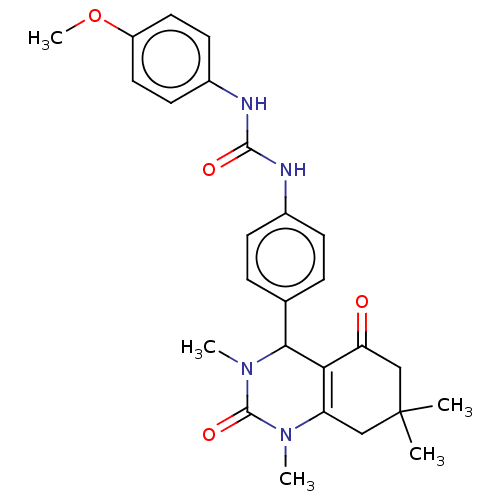 (1-(4-Methoxyphenyl)-3-(4-(1,3,7,7-tetramethyl-5-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246509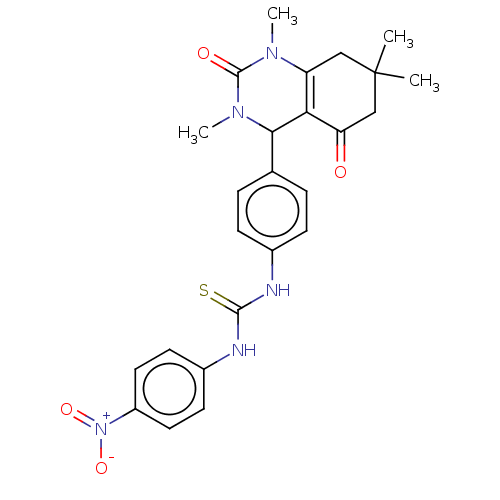 (1-(4-Nitrophenyl)-3-(4-(1,3,7,7-tetramethyl-5-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246506 (1-(4-Methoxyphenyl)-3-(4-(1,3,7,7-tetramethyl-5-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246511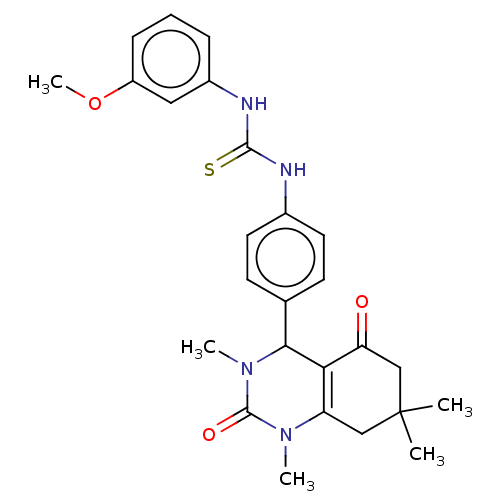 (1-(3-Methoxyphenyl)-3-(4-(1,3,7,7-tetramethyl-5-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM246511 (1-(3-Methoxyphenyl)-3-(4-(1,3,7,7-tetramethyl-5-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM246509 (1-(4-Nitrophenyl)-3-(4-(1,3,7,7-tetramethyl-5-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sakarya University | Assay Description For the inhibition studies of sulphanilamide, different concentrationsof these compounds were added to the enzyme. Activity percentage values of CA f... | J Enzyme Inhib Med Chem 29: 18-22 (2014) Article DOI: 10.3109/14756366.2012.746972 BindingDB Entry DOI: 10.7270/Q28S4NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||