

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429171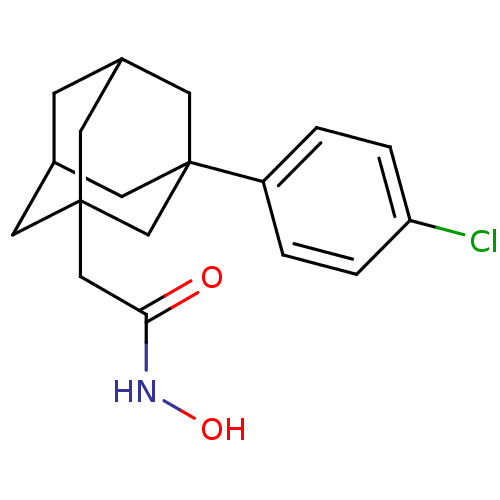 (CHEMBL2336714) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429170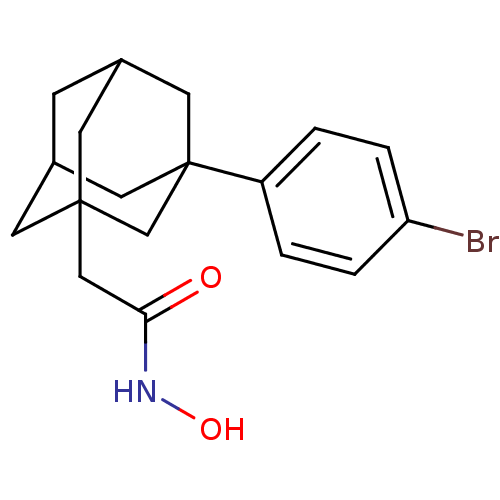 (CHEMBL2336715) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429172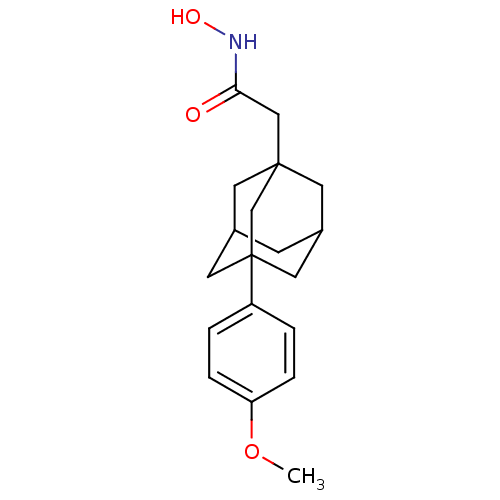 (CHEMBL2336713) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429169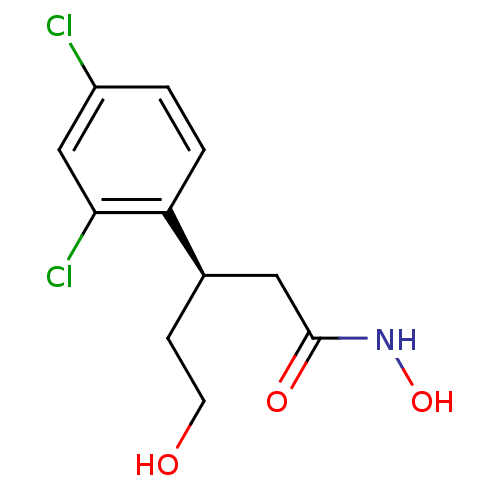 (CHEMBL2336719) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429174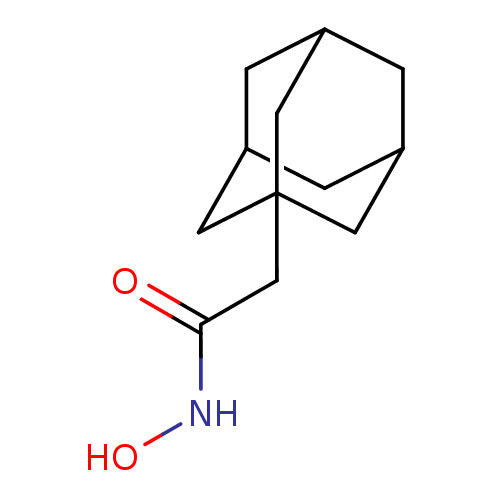 (1-Adamantyl N-Hydroxyacetamide | CHEMBL2336721) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429173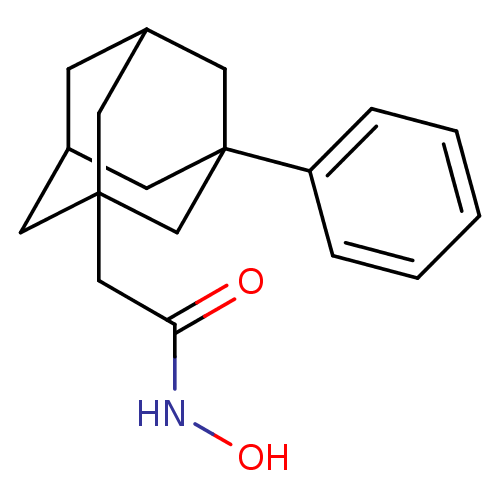 (CHEMBL2336709) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429168 (CHEMBL2336720) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429171 (CHEMBL2336714) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429170 (CHEMBL2336715) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429175 (CHEMBL2336718) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429172 (CHEMBL2336713) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429178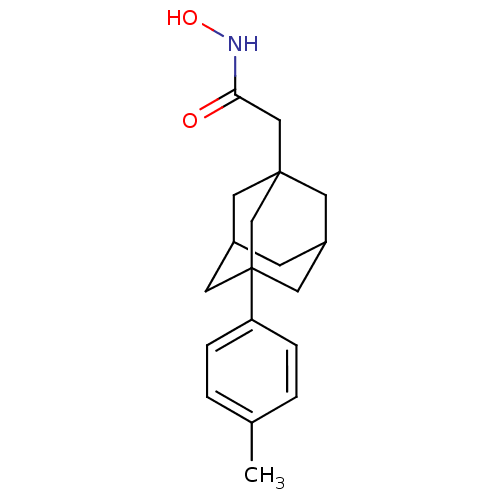 (CHEMBL2336712) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429180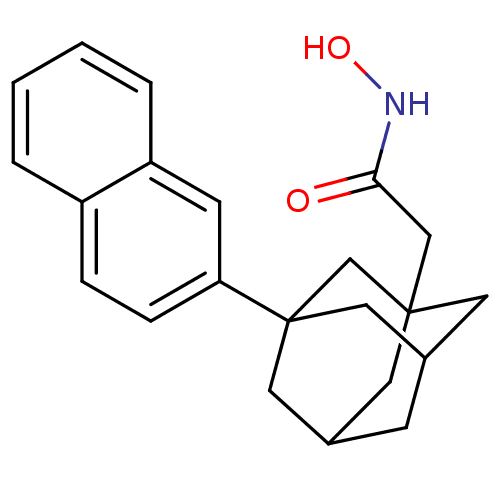 (CHEMBL2336710) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429176 (CHEMBL2336717) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429177 (CHEMBL2336716) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429173 (CHEMBL2336709) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429174 (1-Adamantyl N-Hydroxyacetamide | CHEMBL2336721) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429179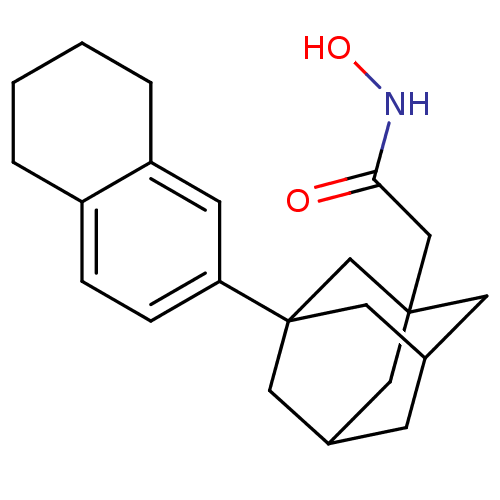 (CHEMBL2336711) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429184 (CHEMBL2336726) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429183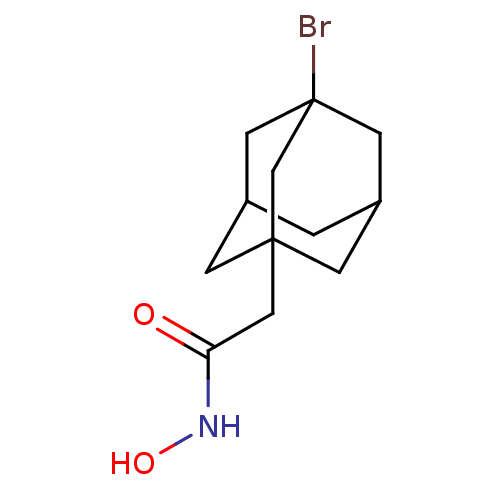 (CHEMBL2336727) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429185 (CHEMBL2336725) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429181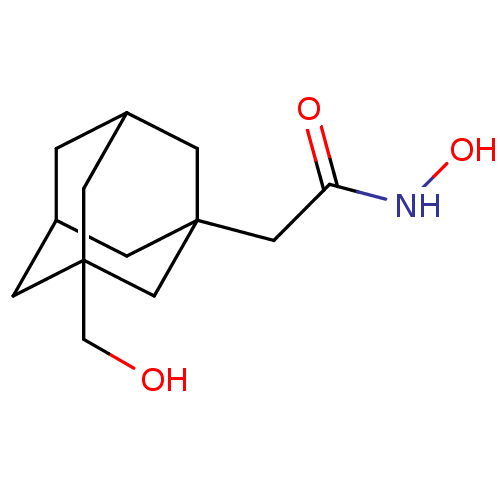 (CHEMBL2336708) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429187 (CHEMBL2336723) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429188 (CHEMBL2336722) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429186 (CHEMBL2336724) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429182 (CHEMBL2336707) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||