

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430535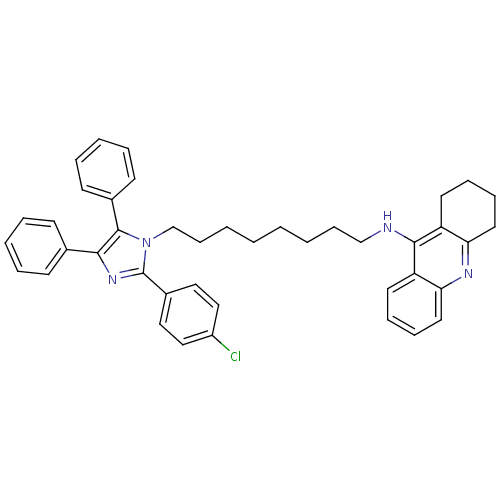 (CHEMBL2336426) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430526 (CHEMBL2335967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430518 (CHEMBL2335975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430534 (CHEMBL2336428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430523 (CHEMBL2335970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430532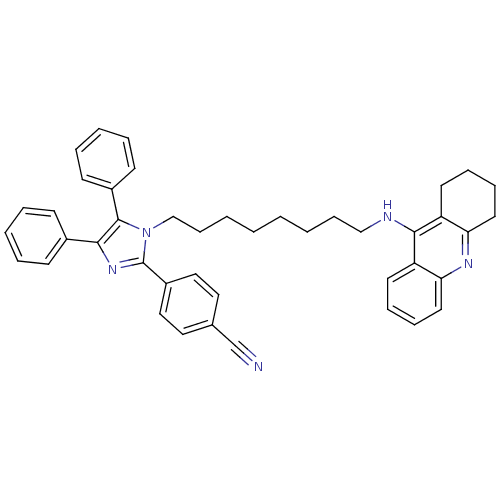 (CHEMBL2336430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430519 (CHEMBL2335974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430522 (CHEMBL2335971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430520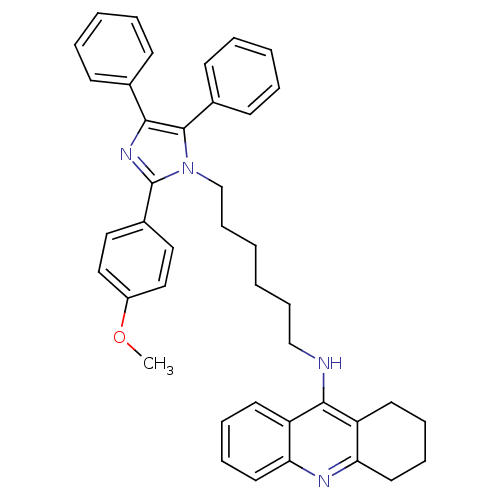 (CHEMBL2335973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430529 (CHEMBL2336432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430528 (CHEMBL2336434) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430536 (CHEMBL2335980) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430521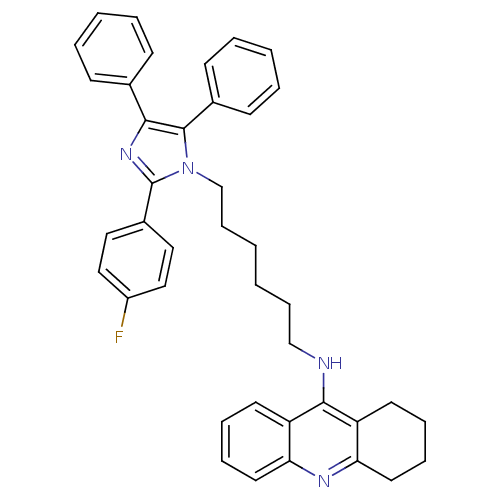 (CHEMBL2335972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430534 (CHEMBL2336428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430524 (CHEMBL2335969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430529 (CHEMBL2336432) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430527 (CHEMBL2335966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430528 (CHEMBL2336434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430527 (CHEMBL2335966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430525 (CHEMBL2335968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430525 (CHEMBL2335968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430530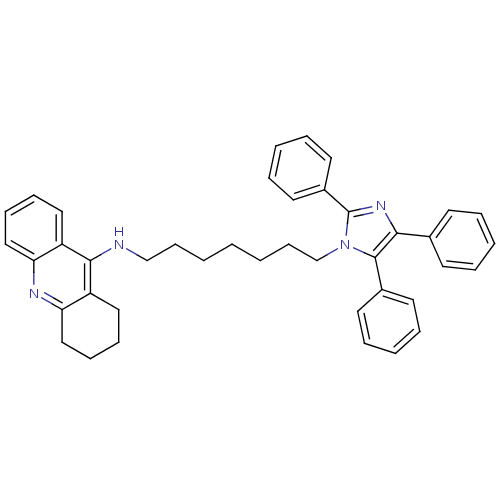 (CHEMBL2336431) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430533 (CHEMBL2336429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430530 (CHEMBL2336431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430535 (CHEMBL2336426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50430531 (CHEMBL2331606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of BuChE in human plasma using butyrylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50430533 (CHEMBL2336429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain cortex using acetylthiocholine iodide as substrate by Ellman's colorimetric method | Eur J Med Chem 62: 556-63 (2013) Article DOI: 10.1016/j.ejmech.2013.01.029 BindingDB Entry DOI: 10.7270/Q25Q4XFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||