 Found 117 hits of Enzyme Inhibition Constant Data
Found 117 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459
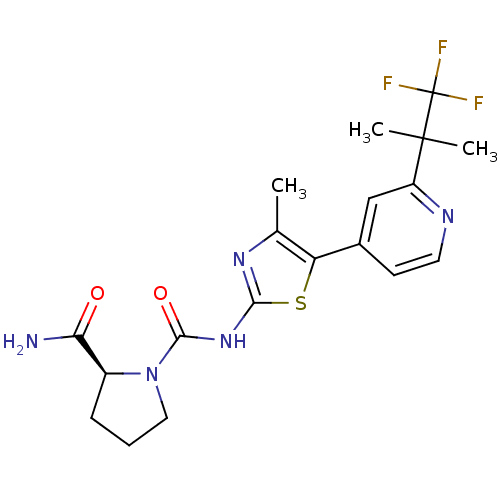
(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448
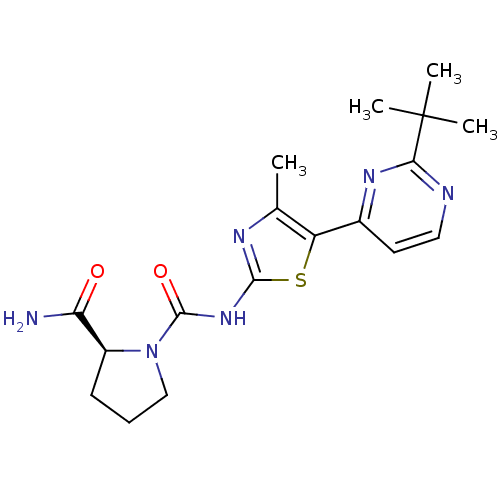
(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436454
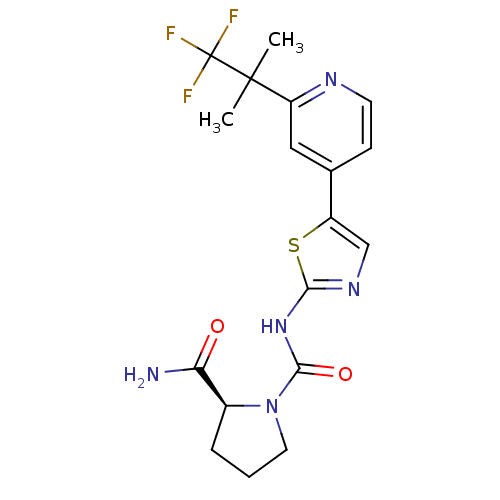
(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436451
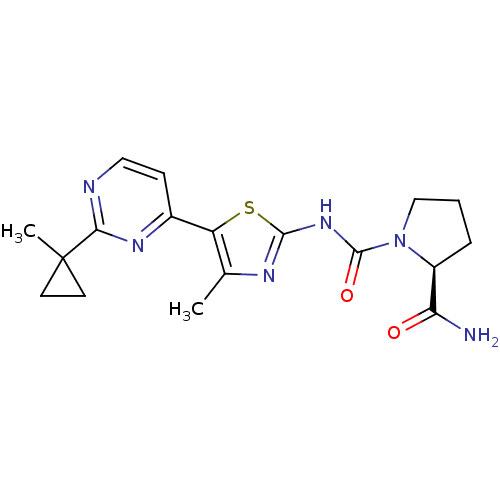
(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436450
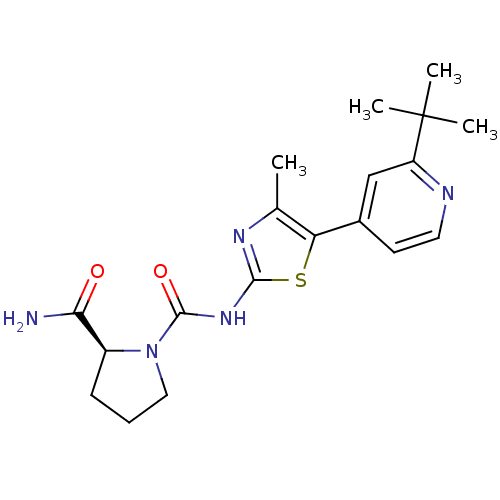
(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436457
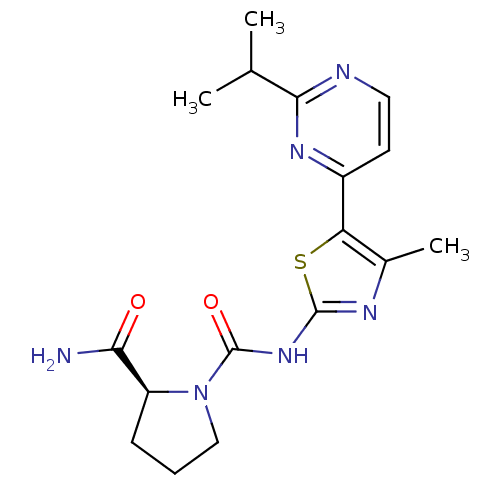
(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436452
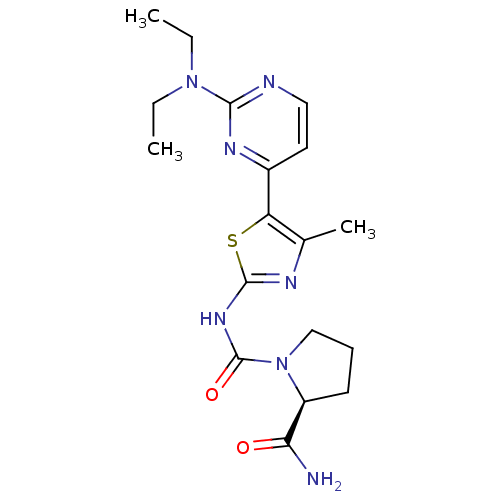
(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436458
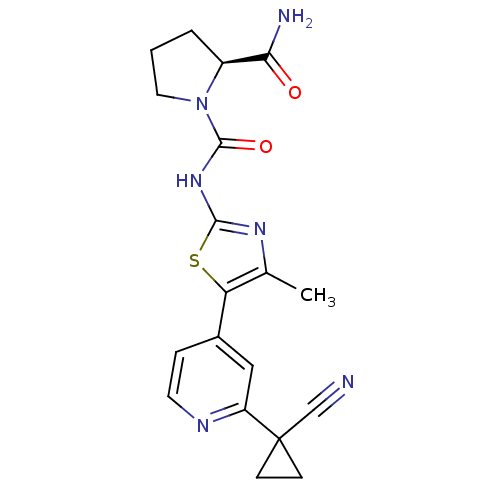
(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436460
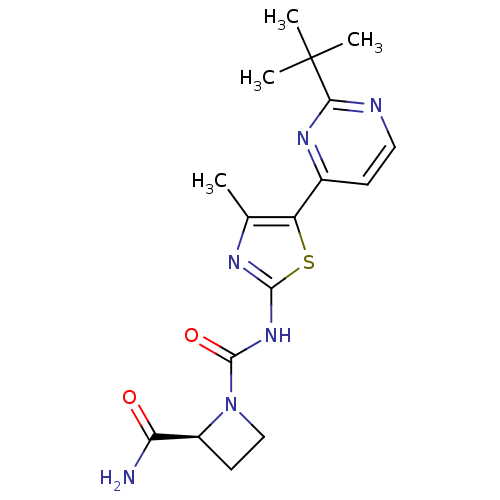
(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436456
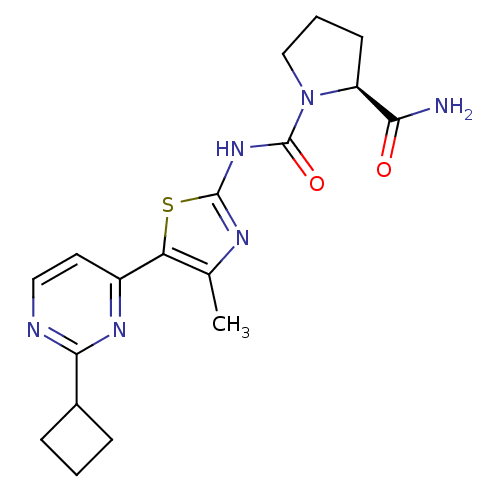
(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436453
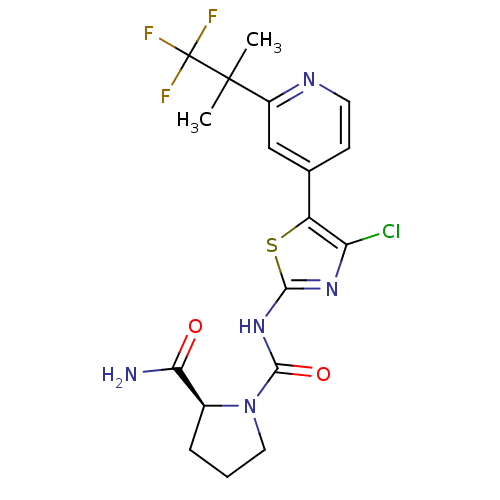
(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
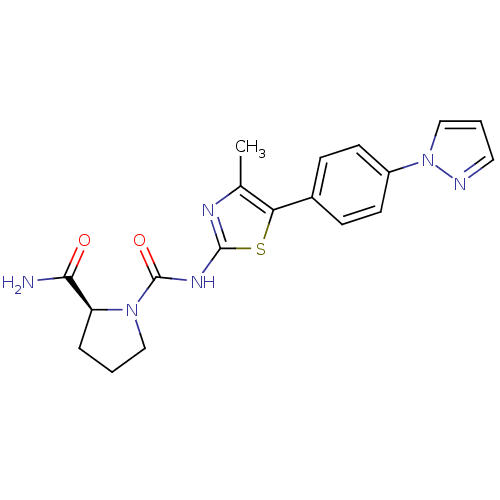
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436452

(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436460

(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436460

(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436455
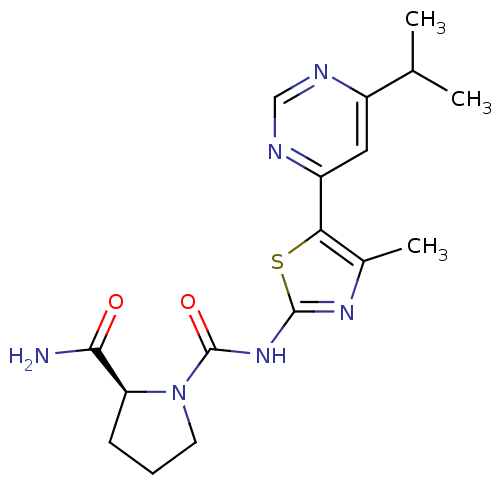
(CHEMBL2397195)Show SMILES CC(C)c1cc(ncn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)11-7-12(20-8-19-11)14-10(3)21-16(26-14)22-17(25)23-6-4-5-13(23)15(18)24/h7-9,13H,4-6H2,1-3H3,(H2,18,24)(H,21,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436460

(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436453

(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436452

(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436452

(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436453

(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436462
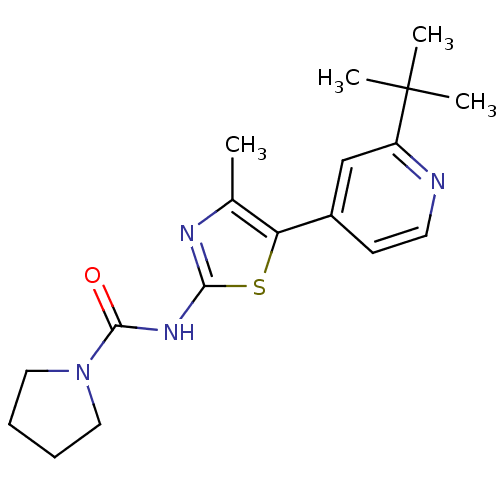
(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390408

(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436462

(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436462

(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436462

(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436452

(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436460

(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436453

(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436460

(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50390408

(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436462

(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436452

(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436449
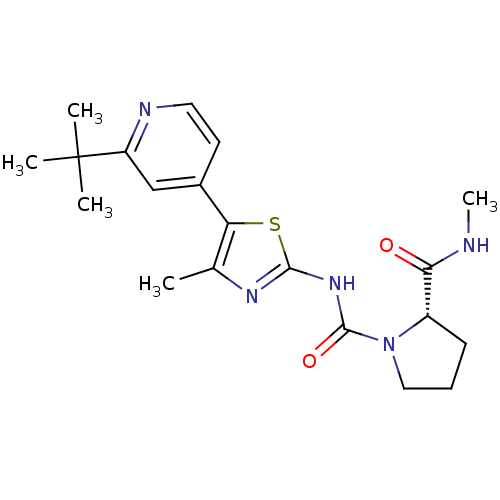
(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436461
(CHEMBL2397190)Show SMILES Cc1nc(NC(=O)N2CCC[C@@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436449

(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436452

(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436455

(CHEMBL2397195)Show SMILES CC(C)c1cc(ncn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)11-7-12(20-8-19-11)14-10(3)21-16(26-14)22-17(25)23-6-4-5-13(23)15(18)24/h7-9,13H,4-6H2,1-3H3,(H2,18,24)(H,21,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436449

(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436453

(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436462

(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436453

(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436449

(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436460

(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436461

(CHEMBL2397190)Show SMILES Cc1nc(NC(=O)N2CCC[C@@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436461

(CHEMBL2397190)Show SMILES Cc1nc(NC(=O)N2CCC[C@@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436449

(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436455

(CHEMBL2397195)Show SMILES CC(C)c1cc(ncn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)11-7-12(20-8-19-11)14-10(3)21-16(26-14)22-17(25)23-6-4-5-13(23)15(18)24/h7-9,13H,4-6H2,1-3H3,(H2,18,24)(H,21,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50436461

(CHEMBL2397190)Show SMILES Cc1nc(NC(=O)N2CCC[C@@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) phosphorylation at Ser1981 by cell-based assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436457

(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of p53 (unknown origin) phosphorylation at Ser15 by cell-based assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436449

(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436462

(CHEMBL2397189)Show InChI InChI=1S/C18H24N4OS/c1-12-15(13-7-8-19-14(11-13)18(2,3)4)24-16(20-12)21-17(23)22-9-5-6-10-22/h7-8,11H,5-6,9-10H2,1-4H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436450

(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436451

(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436449

(CHEMBL2397188)Show SMILES CNC(=O)[C@@H]1CCCN1C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C20H27N5O2S/c1-12-16(13-8-9-22-15(11-13)20(2,3)4)28-18(23-12)24-19(27)25-10-6-7-14(25)17(26)21-5/h8-9,11,14H,6-7,10H2,1-5H3,(H,21,26)(H,23,24,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436455

(CHEMBL2397195)Show SMILES CC(C)c1cc(ncn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)11-7-12(20-8-19-11)14-10(3)21-16(26-14)22-17(25)23-6-4-5-13(23)15(18)24/h7-9,13H,4-6H2,1-3H3,(H2,18,24)(H,21,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436454

(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436455

(CHEMBL2397195)Show SMILES CC(C)c1cc(ncn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)11-7-12(20-8-19-11)14-10(3)21-16(26-14)22-17(25)23-6-4-5-13(23)15(18)24/h7-9,13H,4-6H2,1-3H3,(H2,18,24)(H,21,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436456

(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50436458

(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50436453

(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data