

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM23419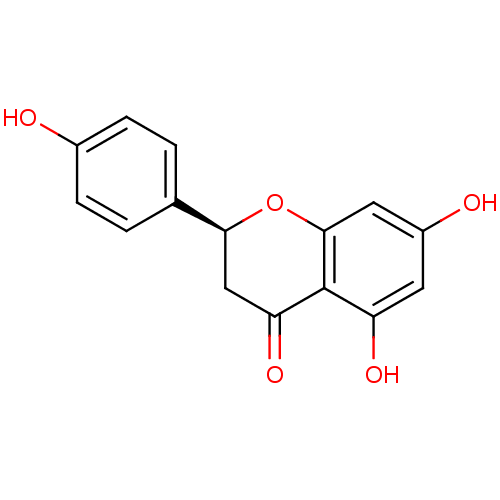 ((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using dibenzylfluorscein as substrate incubated with NADP+ for 10 mins prior to substrate addition measured ... | Eur J Med Chem 68: 412-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.052 BindingDB Entry DOI: 10.7270/Q2765GSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50441056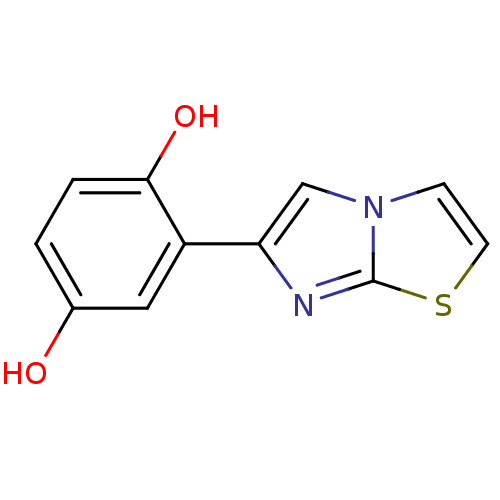 (CHEMBL2430196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using dibenzylfluorscein as substrate incubated with NADP+ for 10 mins prior to substrate addition measured ... | Eur J Med Chem 68: 412-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.052 BindingDB Entry DOI: 10.7270/Q2765GSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50441057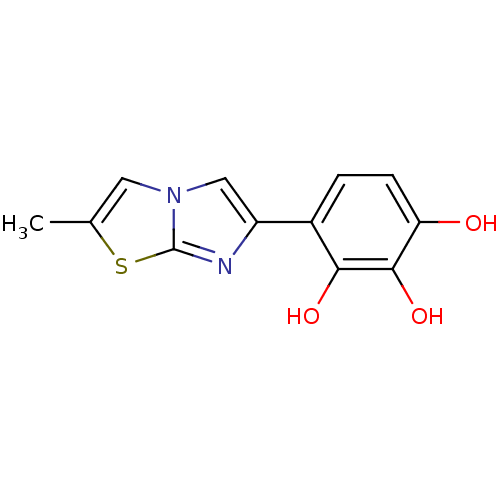 (CHEMBL2430197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using dibenzylfluorscein as substrate incubated with NADP+ for 10 mins prior to substrate addition measured ... | Eur J Med Chem 68: 412-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.052 BindingDB Entry DOI: 10.7270/Q2765GSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50441056 (CHEMBL2430196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in mouse RAW264.7 cells by Griess assay | Eur J Med Chem 68: 412-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.052 BindingDB Entry DOI: 10.7270/Q2765GSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50441057 (CHEMBL2430197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in mouse RAW264.7 cells by Griess assay | Eur J Med Chem 68: 412-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.052 BindingDB Entry DOI: 10.7270/Q2765GSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50441055 (CHEMBL1744478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in mouse RAW264.7 cells by Griess assay | Eur J Med Chem 68: 412-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.052 BindingDB Entry DOI: 10.7270/Q2765GSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||