

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444240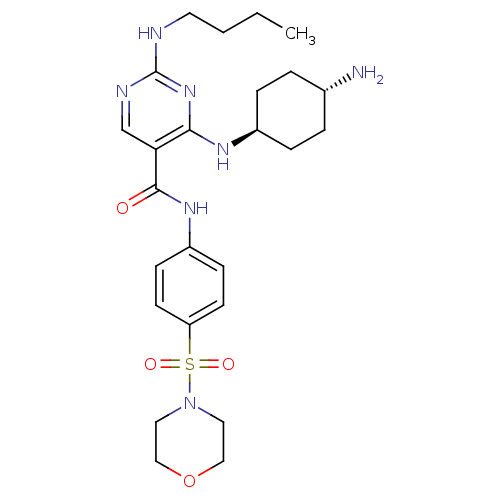 (CHEMBL3093635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444253 (CHEMBL3093648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444241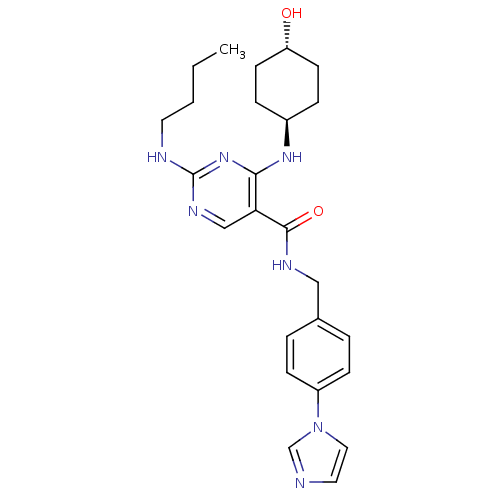 (CHEMBL3093756 | US9649309, Compound UNC2881A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444242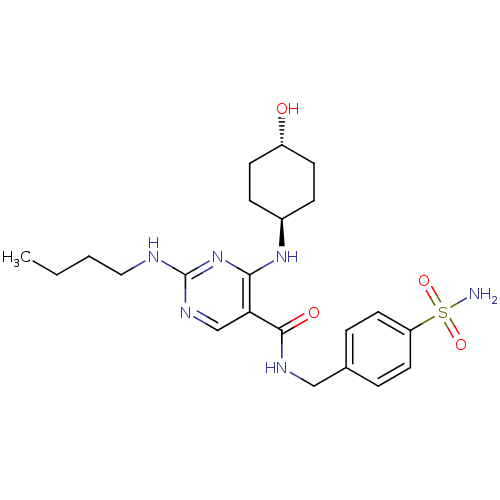 (CHEMBL3093755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444244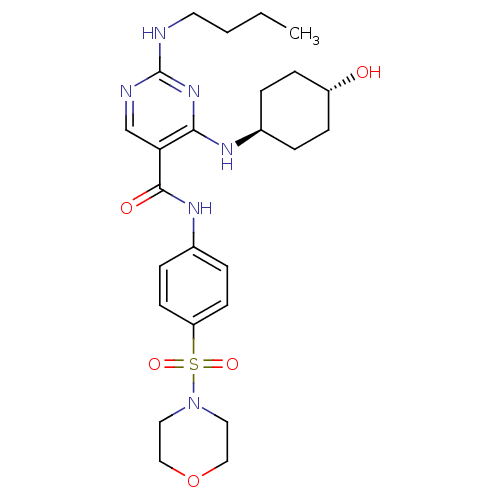 (CHEMBL3093753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444245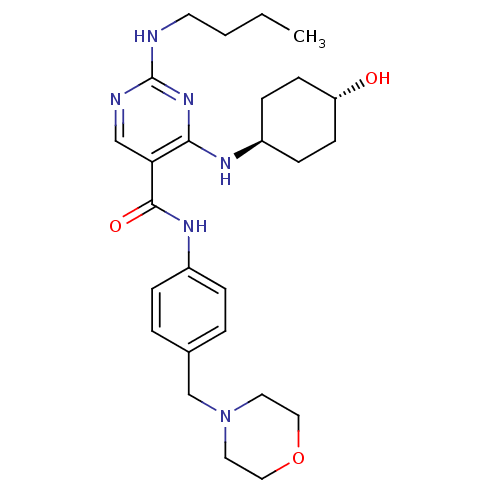 (CHEMBL3093752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444271 (CHEMBL3093627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444251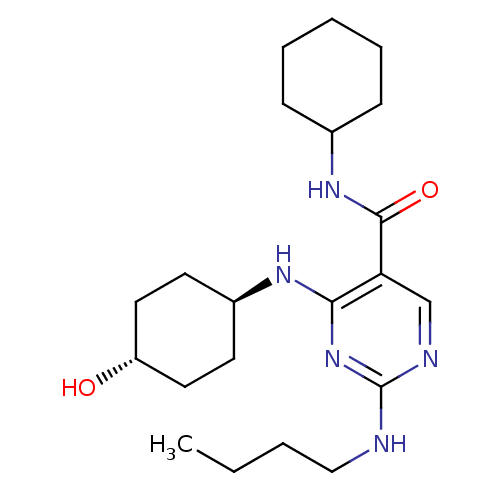 (CHEMBL3093746 | US9649309, Compound UNC2602A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444254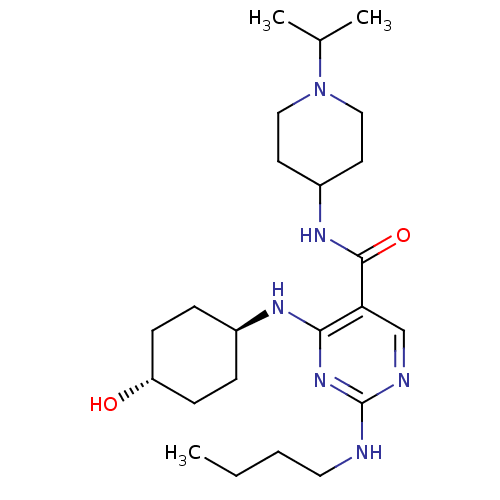 (CHEMBL3093763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444256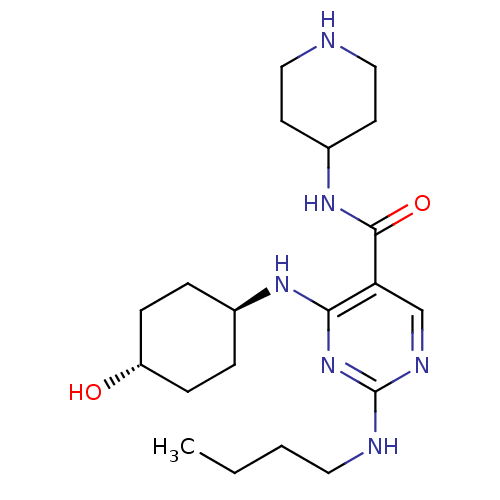 (CHEMBL3093761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444273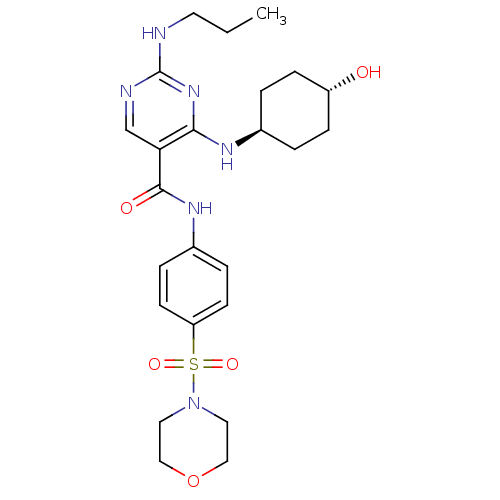 (CHEMBL3093625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444252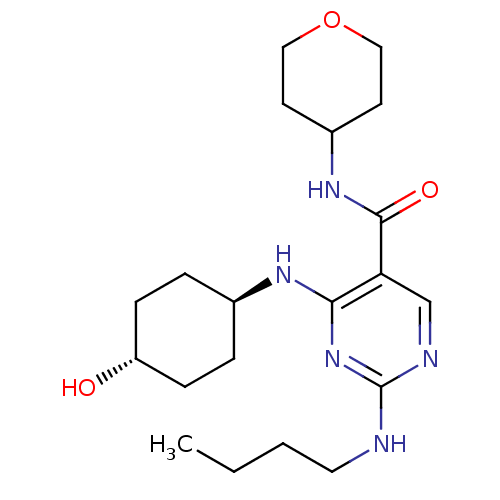 (CHEMBL3093649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444246 (CHEMBL3093751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444243 (CHEMBL3093754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444249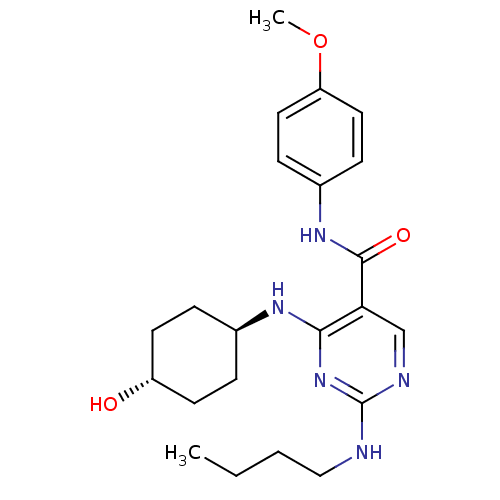 (CHEMBL3093748 | US9649309, Compound UNC2776A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444241 (CHEMBL3093756 | US9649309, Compound UNC2881A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase phosphorylation in human 697 B-ALL cells after 1 hr by Western blot analysis | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444260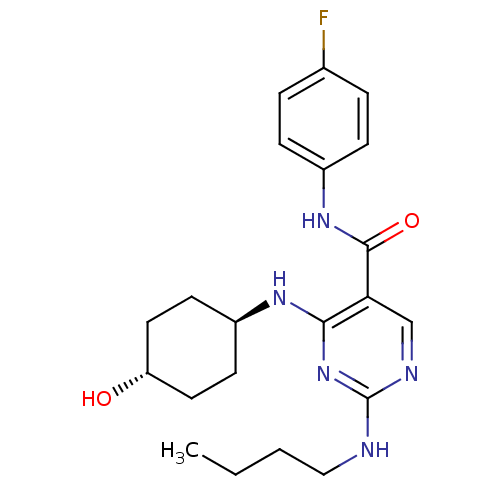 (CHEMBL3093757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444268 (CHEMBL3093630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444272 (CHEMBL3093626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444248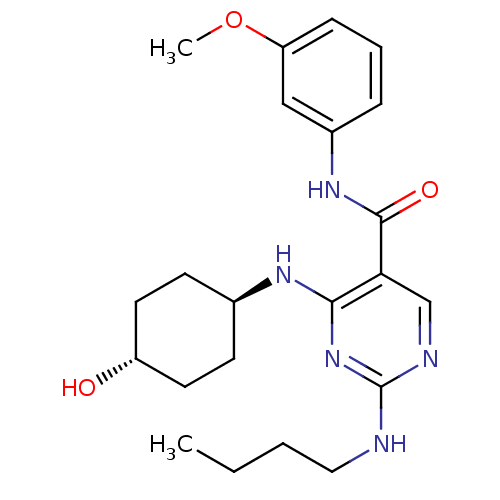 (CHEMBL3093749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444274 (CHEMBL3093647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444284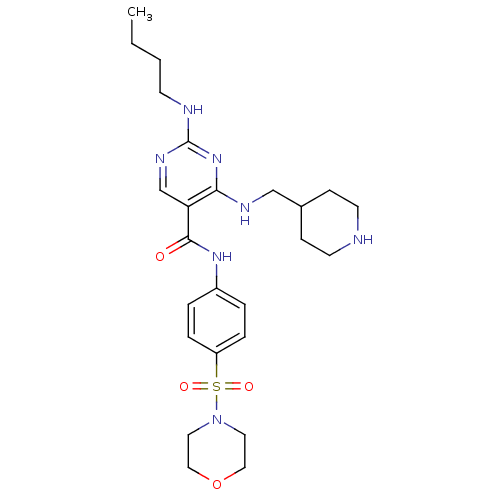 (CHEMBL3093637 | US9649309, Compound UNC2084A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444247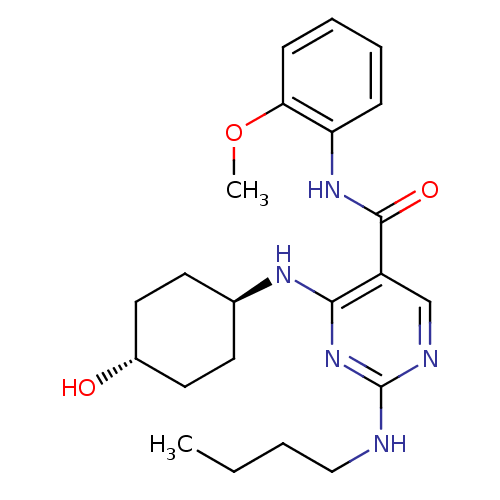 (CHEMBL3093750 | US9649309, Compound UNC2775A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444262 (CHEMBL3093764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444267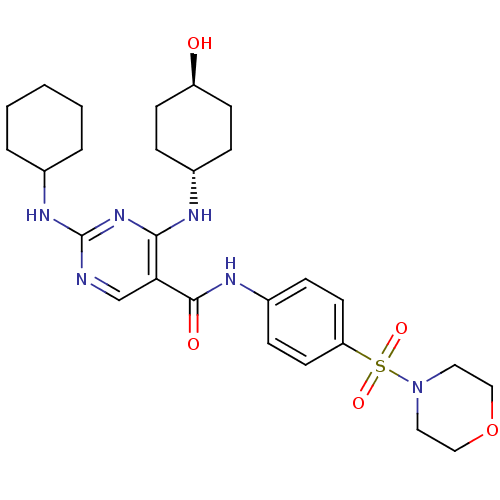 (CHEMBL3093631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444269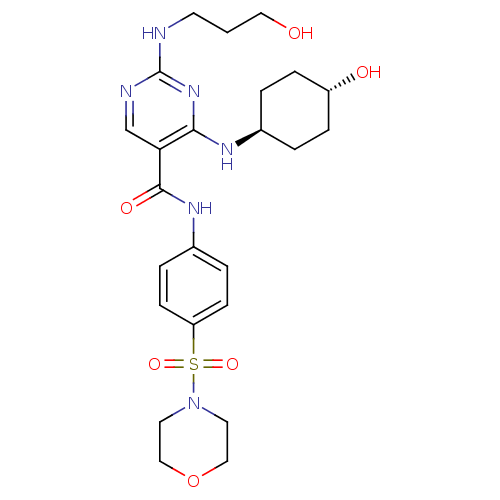 (CHEMBL3093629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444282 (CHEMBL3093639 | US9649309, Compound UNC2086A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444258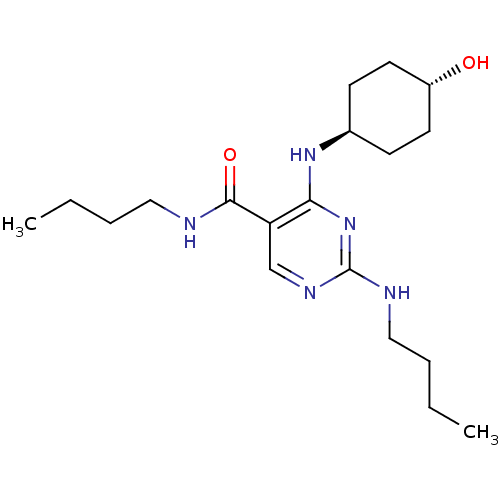 (CHEMBL3093759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444270 (CHEMBL3093628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444280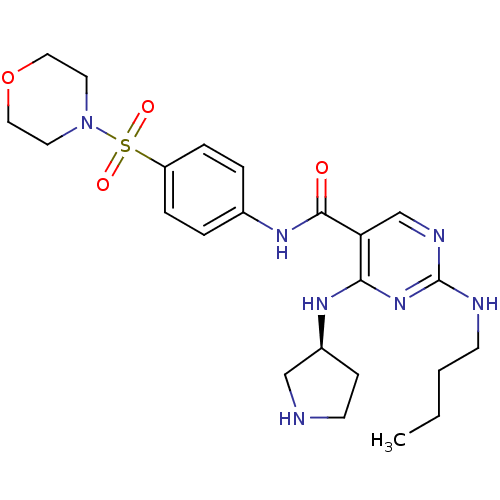 (CHEMBL3093641 | US9649309, Compound UNC2091A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444265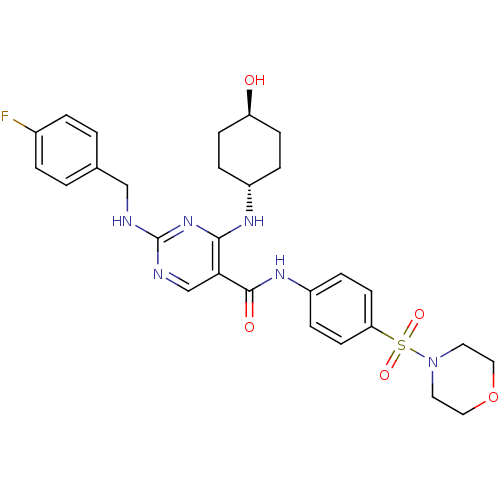 (CHEMBL3093633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444283 (CHEMBL3093638 | US9649309, Compound UNC2090A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444275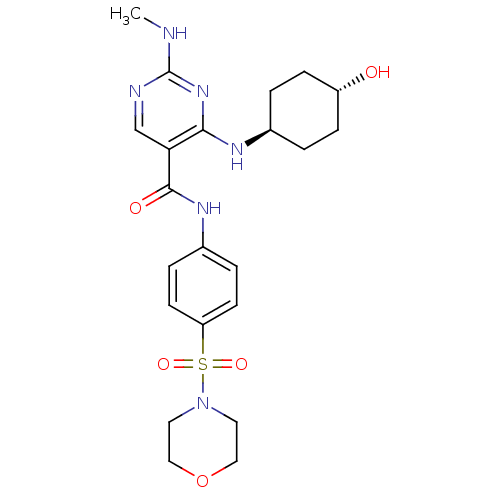 (CHEMBL3093646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444278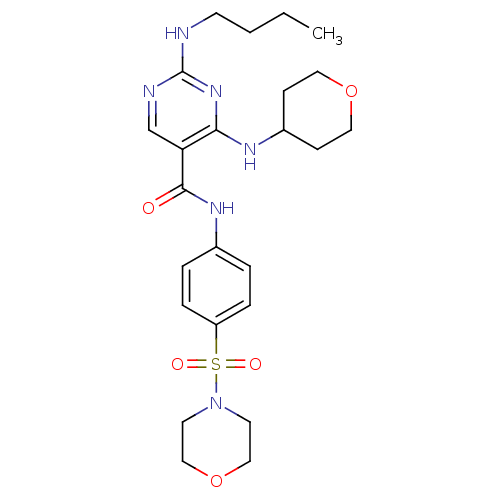 (CHEMBL3093643 | US9649309, Compound UNC2061A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444266 (CHEMBL3093632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444254 (CHEMBL3093763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444250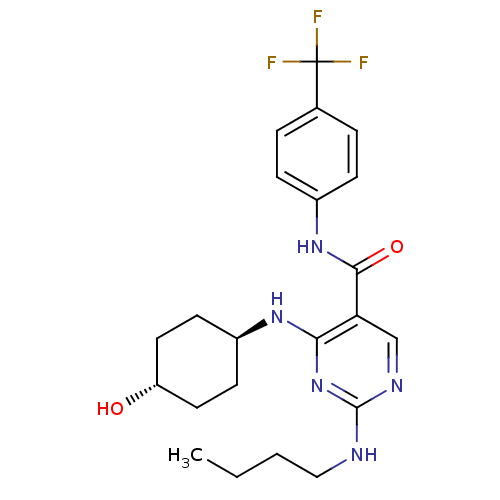 (CHEMBL3093747 | US9649309, Compound UNC2803A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444245 (CHEMBL3093752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444241 (CHEMBL3093756 | US9649309, Compound UNC2881A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444242 (CHEMBL3093755) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444242 (CHEMBL3093755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444271 (CHEMBL3093627) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444241 (CHEMBL3093756 | US9649309, Compound UNC2881A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444245 (CHEMBL3093752) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444251 (CHEMBL3093746 | US9649309, Compound UNC2602A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444251 (CHEMBL3093746 | US9649309, Compound UNC2602A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444240 (CHEMBL3093635) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444240 (CHEMBL3093635) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444254 (CHEMBL3093763) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444246 (CHEMBL3093751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444253 (CHEMBL3093648) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444256 (CHEMBL3093761) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444244 (CHEMBL3093753) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444256 (CHEMBL3093761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444252 (CHEMBL3093649) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444253 (CHEMBL3093648) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444243 (CHEMBL3093754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444246 (CHEMBL3093751) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444252 (CHEMBL3093649) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444285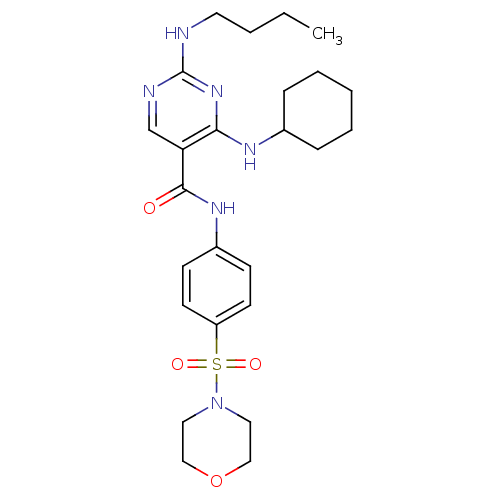 (CHEMBL3093636 | US9649309, Compound UNC2082A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444273 (CHEMBL3093625) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444279 (CHEMBL3093642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444284 (CHEMBL3093637 | US9649309, Compound UNC2084A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444249 (CHEMBL3093748 | US9649309, Compound UNC2776A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444244 (CHEMBL3093753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444274 (CHEMBL3093647) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444243 (CHEMBL3093754) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444248 (CHEMBL3093749) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444249 (CHEMBL3093748 | US9649309, Compound UNC2776A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444262 (CHEMBL3093764) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444271 (CHEMBL3093627) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444281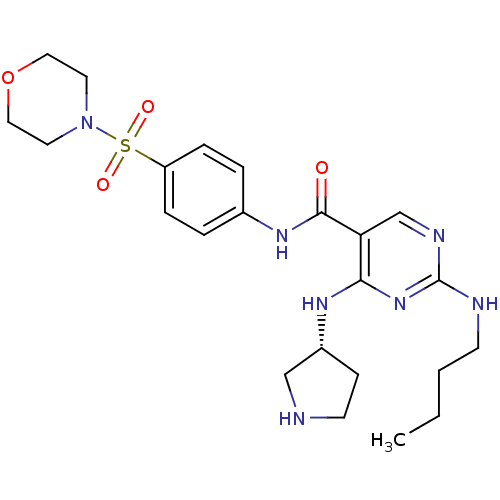 (CHEMBL3093640 | US9649309, Compound UNC2089A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444272 (CHEMBL3093626) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444275 (CHEMBL3093646) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444273 (CHEMBL3093625) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444247 (CHEMBL3093750 | US9649309, Compound UNC2775A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444283 (CHEMBL3093638 | US9649309, Compound UNC2090A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444268 (CHEMBL3093630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444248 (CHEMBL3093749) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444260 (CHEMBL3093757) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444268 (CHEMBL3093630) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444280 (CHEMBL3093641 | US9649309, Compound UNC2091A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444282 (CHEMBL3093639 | US9649309, Compound UNC2086A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444262 (CHEMBL3093764) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444260 (CHEMBL3093757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444266 (CHEMBL3093632) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444276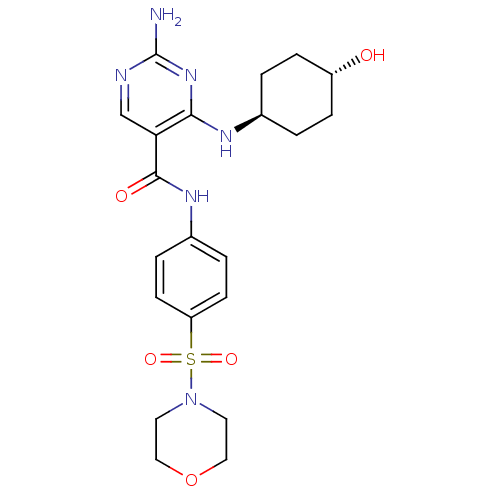 (CHEMBL3093645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444255 (CHEMBL3093762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444258 (CHEMBL3093759) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444247 (CHEMBL3093750 | US9649309, Compound UNC2775A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444279 (CHEMBL3093642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444269 (CHEMBL3093629) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444267 (CHEMBL3093631) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444284 (CHEMBL3093637 | US9649309, Compound UNC2084A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444270 (CHEMBL3093628) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444270 (CHEMBL3093628) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444257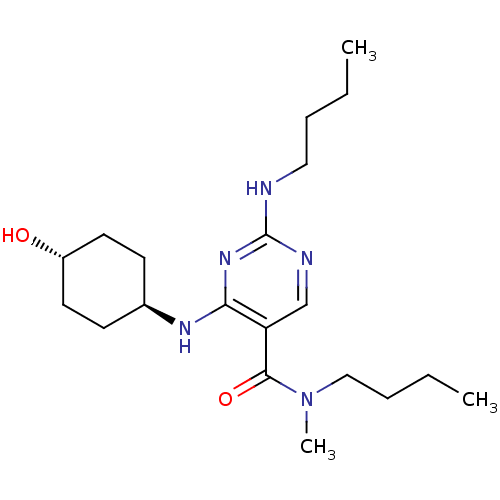 (CHEMBL3093760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444258 (CHEMBL3093759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444269 (CHEMBL3093629) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444266 (CHEMBL3093632) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444267 (CHEMBL3093631) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444277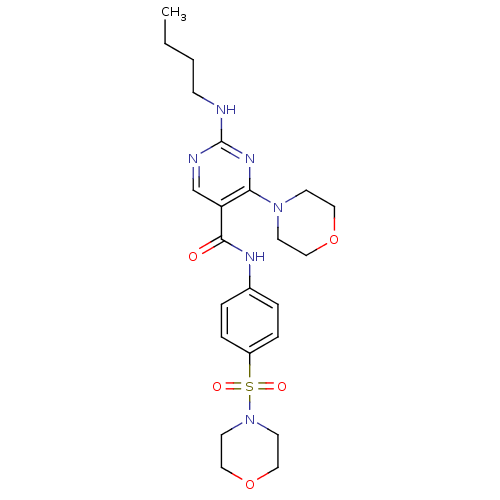 (CHEMBL3093644 | US9649309, Compound UNC2081A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444282 (CHEMBL3093639 | US9649309, Compound UNC2086A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444280 (CHEMBL3093641 | US9649309, Compound UNC2091A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444261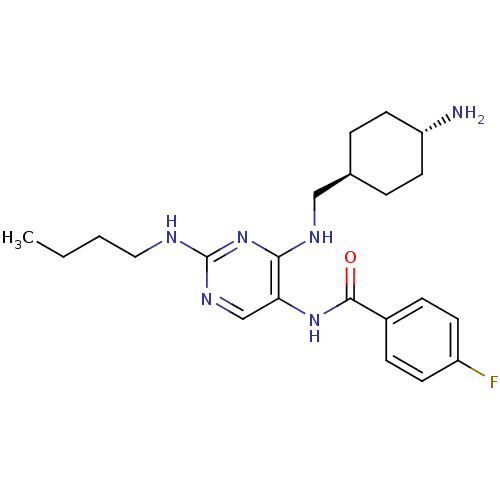 (CHEMBL3093765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444283 (CHEMBL3093638 | US9649309, Compound UNC2090A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444274 (CHEMBL3093647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444250 (CHEMBL3093747 | US9649309, Compound UNC2803A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444276 (CHEMBL3093645) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444250 (CHEMBL3093747 | US9649309, Compound UNC2803A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444281 (CHEMBL3093640 | US9649309, Compound UNC2089A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444279 (CHEMBL3093642) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444272 (CHEMBL3093626) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444259 (CHEMBL3093758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444277 (CHEMBL3093644 | US9649309, Compound UNC2081A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444264 (CHEMBL3093634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444257 (CHEMBL3093760) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444263 (CHEMBL3093624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444263 (CHEMBL3093624) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444281 (CHEMBL3093640 | US9649309, Compound UNC2089A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444265 (CHEMBL3093633) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444261 (CHEMBL3093765) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444259 (CHEMBL3093758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444278 (CHEMBL3093643 | US9649309, Compound UNC2061A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444261 (CHEMBL3093765) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444264 (CHEMBL3093634) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444265 (CHEMBL3093633) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444285 (CHEMBL3093636 | US9649309, Compound UNC2082A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444275 (CHEMBL3093646) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444277 (CHEMBL3093644 | US9649309, Compound UNC2081A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444276 (CHEMBL3093645) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444255 (CHEMBL3093762) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444285 (CHEMBL3093636 | US9649309, Compound UNC2082A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444263 (CHEMBL3093624) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444257 (CHEMBL3093760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444255 (CHEMBL3093762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444278 (CHEMBL3093643 | US9649309, Compound UNC2061A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444259 (CHEMBL3093758) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444264 (CHEMBL3093634) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9693-700 (2014) Article DOI: 10.1021/jm4013888 BindingDB Entry DOI: 10.7270/Q21G0NQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||