

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446495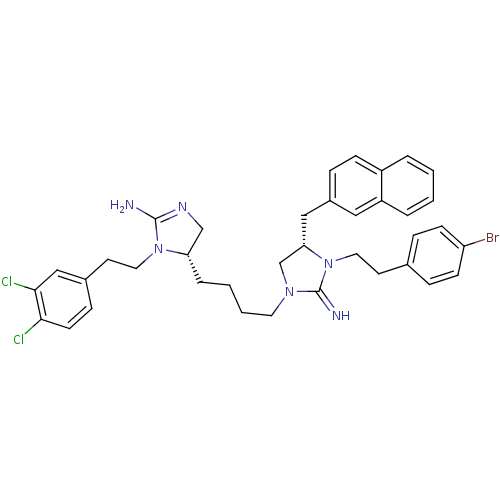 (CHEMBL3110029) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446499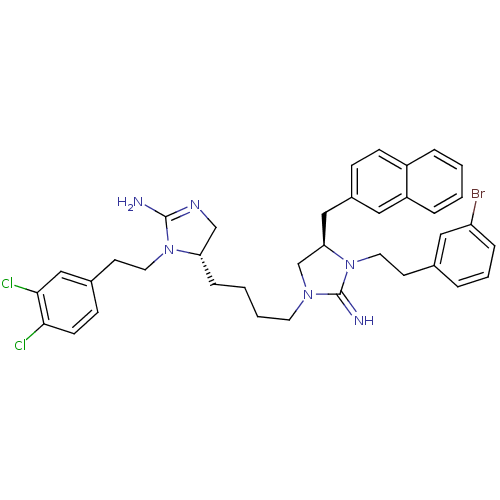 (CHEMBL3110025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446498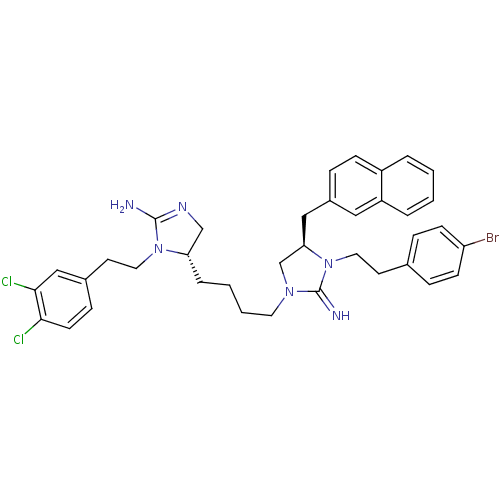 (CHEMBL3110026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/alpha-5/beta-4 (Homo sapiens (Human)) | BDBM50446500 (CHEMBL3110043) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Binding affinity to alpha3beta4alpha5 nAChR (unknown origin) | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446501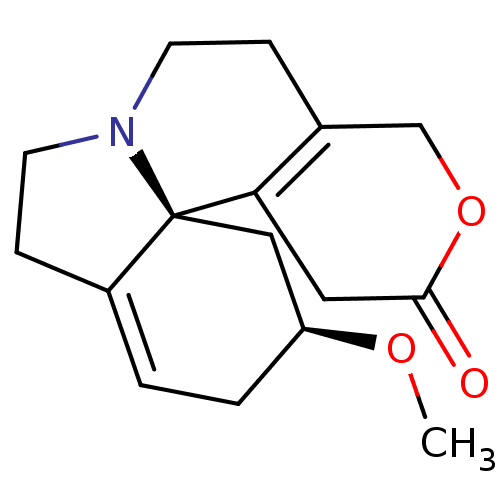 (CHEMBL1319741 | Dihydro-Beta-Erythroidine Hydrobro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446485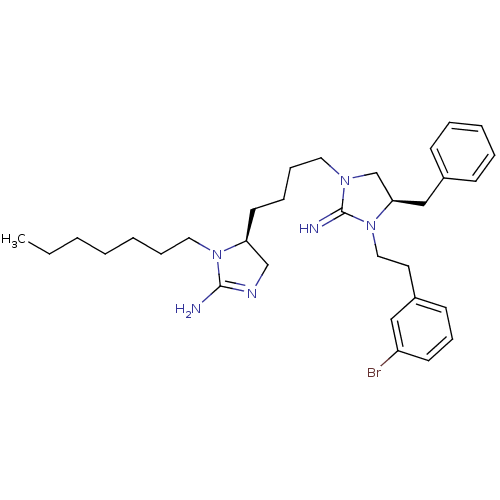 (CHEMBL3110039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446486 (CHEMBL3110038) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446487 (CHEMBL3110037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446484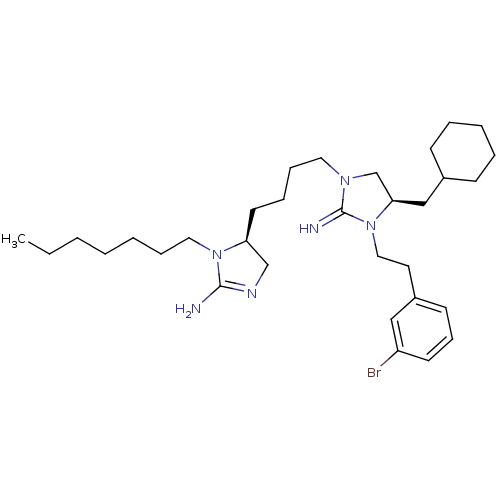 (CHEMBL3110040) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446508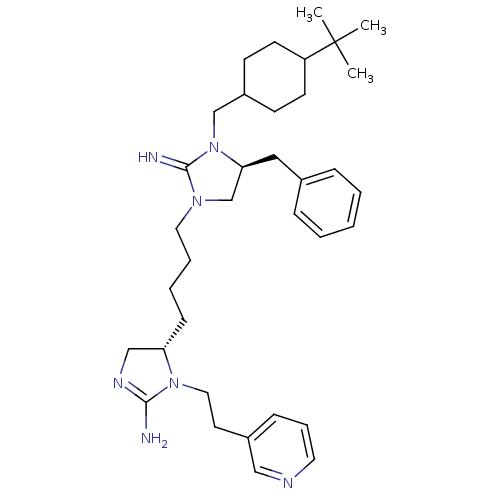 (CHEMBL3110044) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446507 (CHEMBL3110045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446502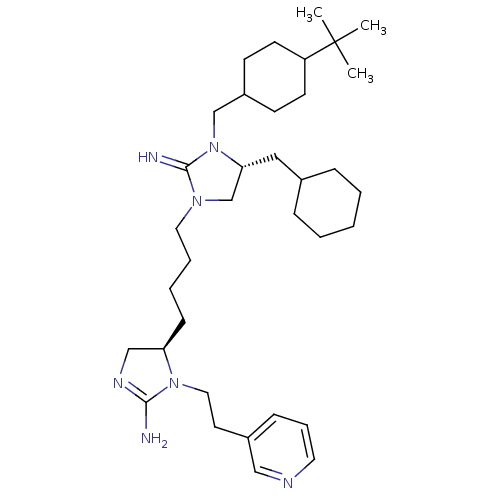 (CHEMBL3110050) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446509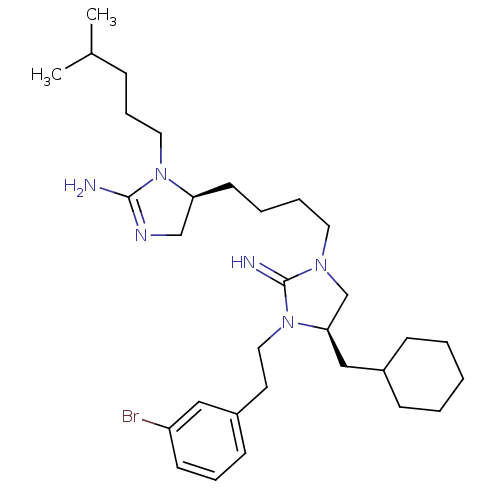 (CHEMBL3110042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446483 (CHEMBL3110041) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446505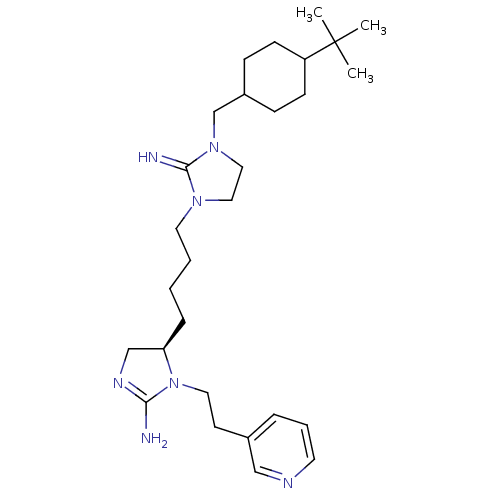 (CHEMBL3110047) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446491 (CHEMBL3110033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446490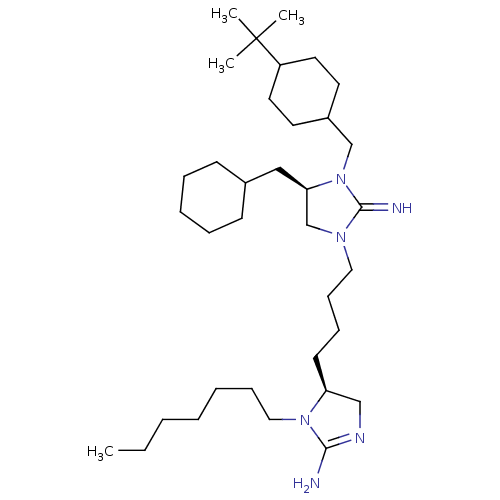 (CHEMBL3110034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446500 (CHEMBL3110043) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446504 (CHEMBL3110048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446493 (CHEMBL3110031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446506 (CHEMBL3110046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446489 (CHEMBL3110035) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446496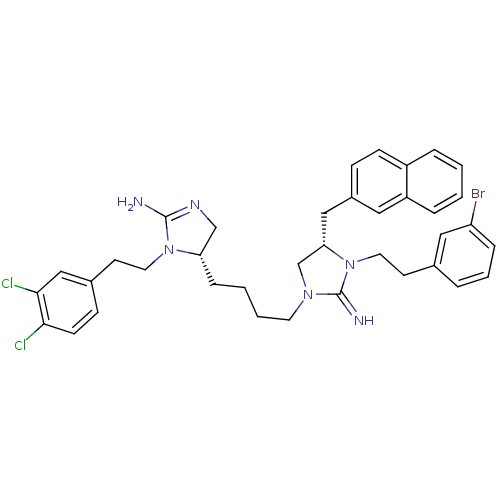 (CHEMBL3110028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446503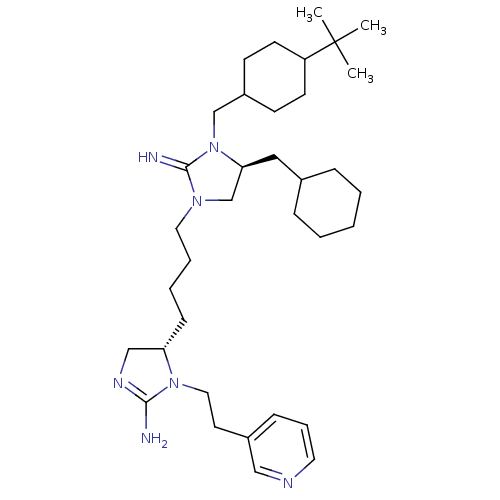 (CHEMBL3110049) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446488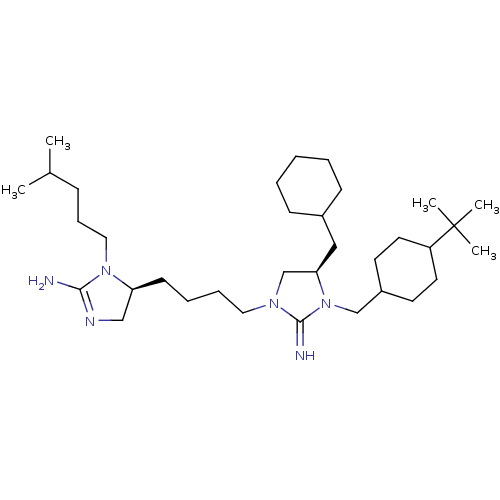 (CHEMBL3110036) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446492 (CHEMBL3110032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446494 (CHEMBL3110030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50446497 (CHEMBL3110027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR transfected in HEK293 cells after 2 hrs by beta-plate counting analysis | J Med Chem 56: 10103-17 (2013) Article DOI: 10.1021/jm401543h BindingDB Entry DOI: 10.7270/Q25D8TBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||