

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of MOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50444943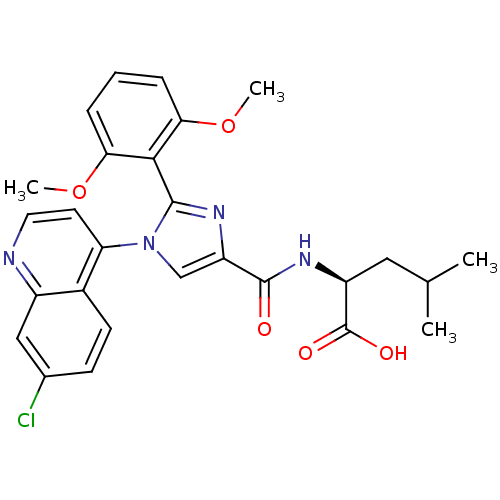 (CHEMBL3099773) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738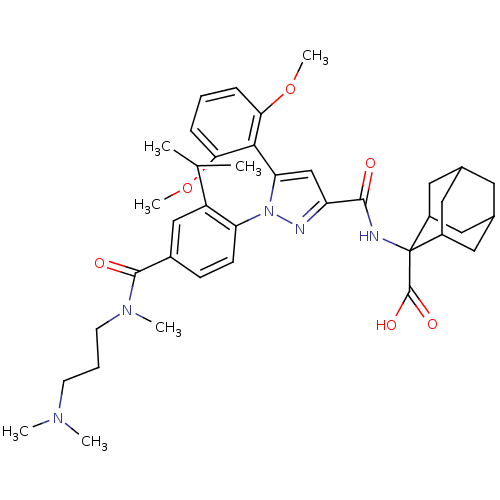 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-neurotensin from NTR1 (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444944 (CHEMBL3099772) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444954 (CHEMBL3099779) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444958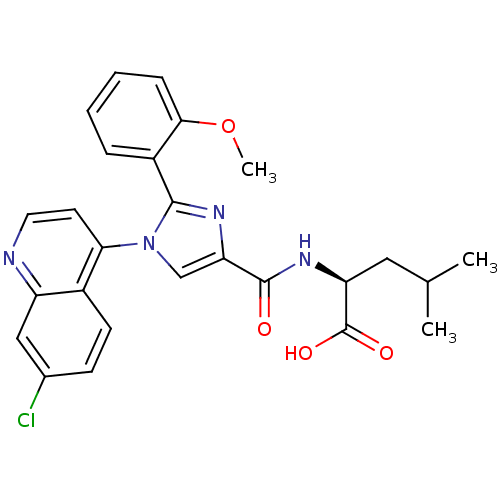 (CHEMBL3099775) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444945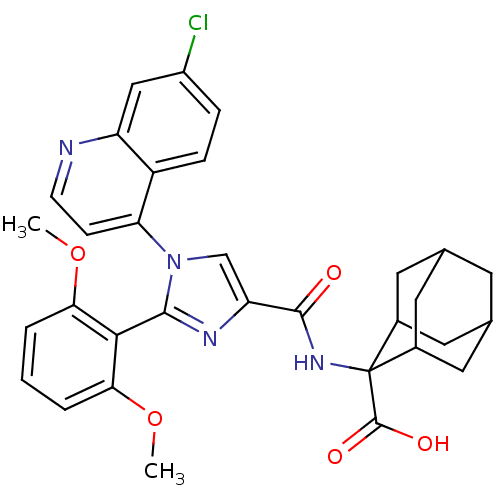 (CHEMBL3099771) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444950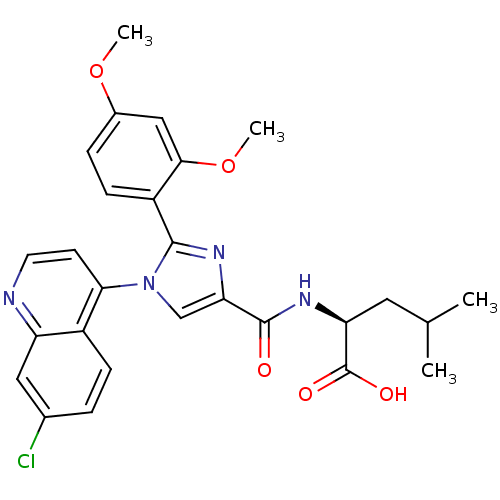 (CHEMBL3099783) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444949 (CHEMBL3099767) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444957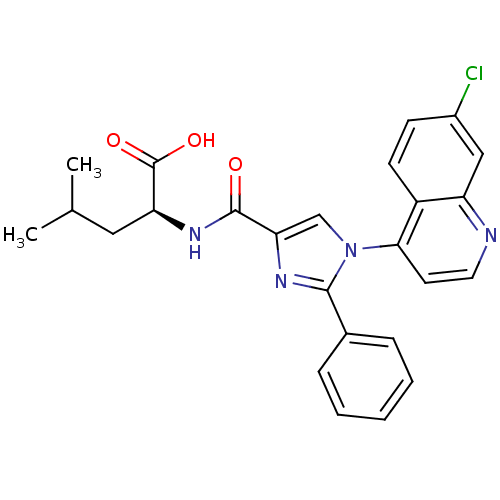 (CHEMBL3099776) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444959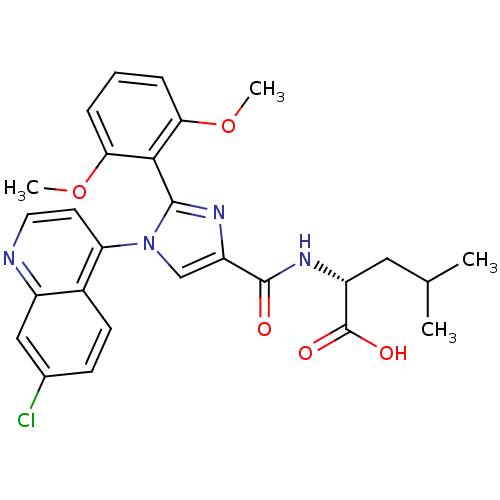 (CHEMBL3099774) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248034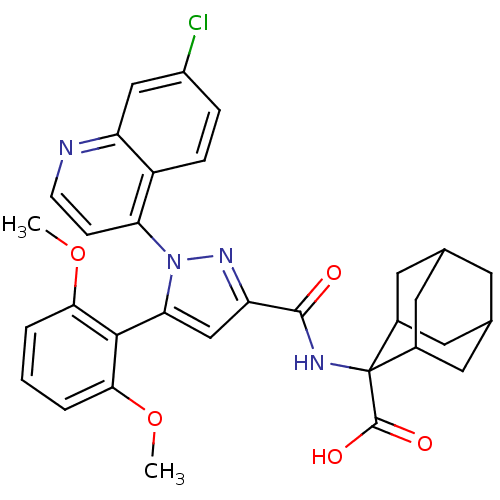 (2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 (unknown origin) expressed in CHO-K1 cells coexpressing beta-arrestin/N-terminal deletion mutant of beta-galactosidase fusio... | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 (unknown origin) expressed in CHO-K1 cells coexpressing beta-arrestin/N-terminal deletion mutant of beta-galactosidase fusio... | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR2 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR2 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 298 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 (unknown origin) expressed in CHO cells assessed as Ca2+ mobilization by Fluo-4 NW dye-based fluorescence assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at GPR35 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444953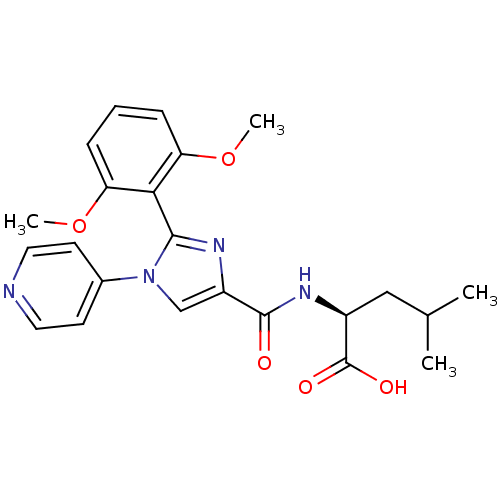 (CHEMBL3099780) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444955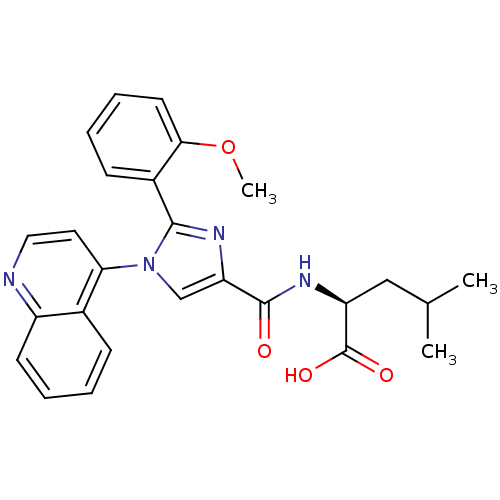 (CHEMBL3099778) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at GPR35 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444951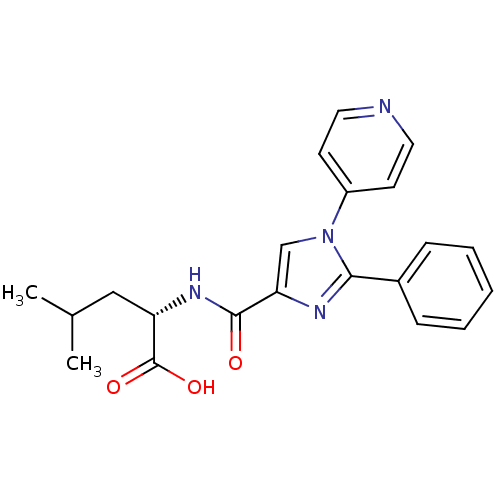 (CHEMBL3099782) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444948 (CHEMBL3099768) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444946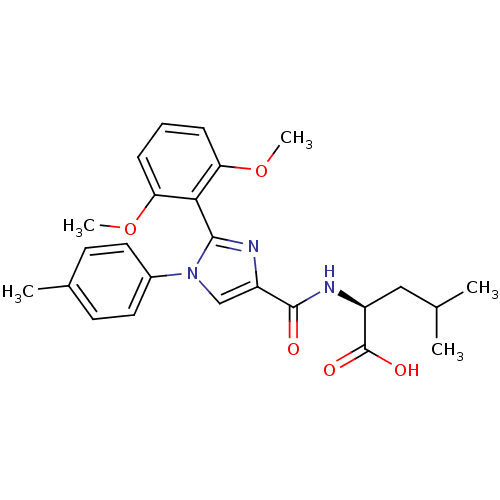 (CHEMBL3099770) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444952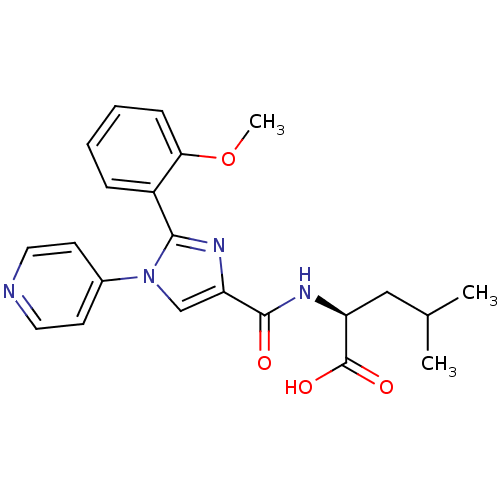 (CHEMBL3099781) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444947 (CHEMBL3099769) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <156 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 (unknown origin) expressed in CHO cells assessed as Ca2+ mobilization by Fluo-4 NW dye-based fluorescence assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444956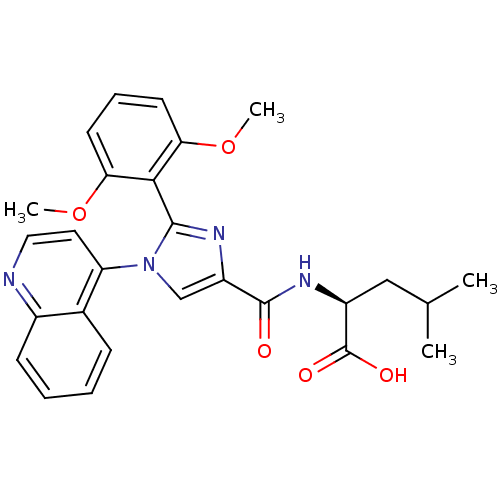 (CHEMBL3099777) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assay | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||