

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448498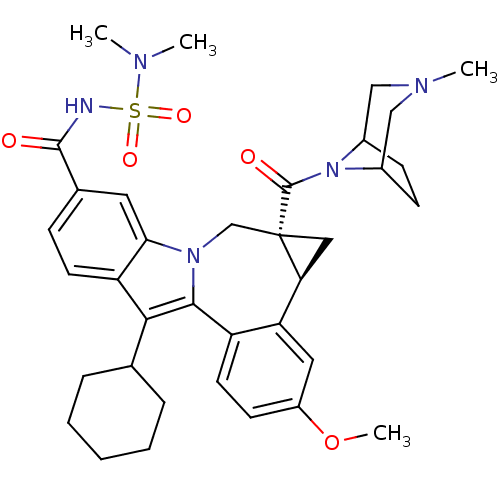 (BMS-791325 | Beclabuvir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50448498 (BMS-791325 | Beclabuvir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448498 (BMS-791325 | Beclabuvir) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50448498 (BMS-791325 | Beclabuvir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50448495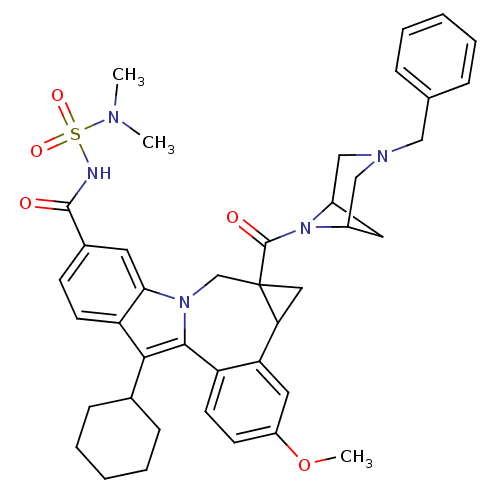 (CHEMBL3126836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448500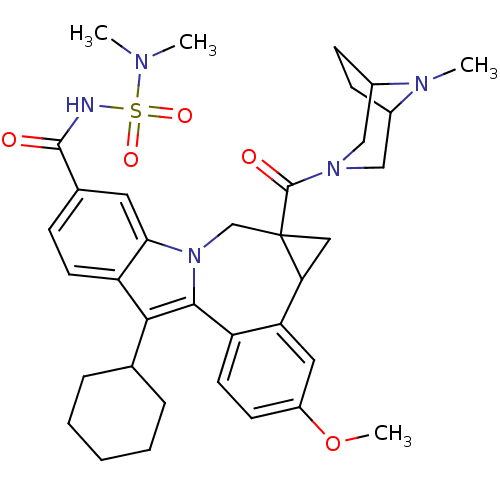 (CHEMBL3126840) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448510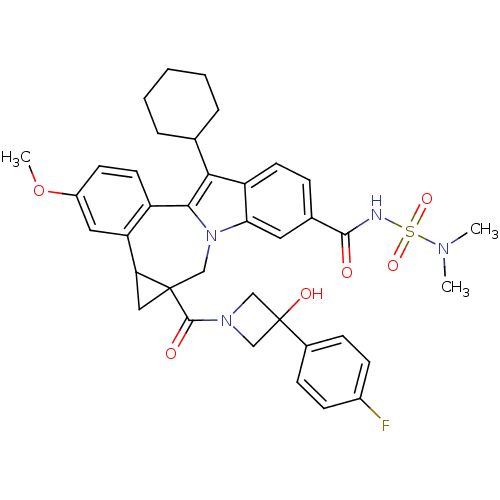 (CHEMBL3126858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448513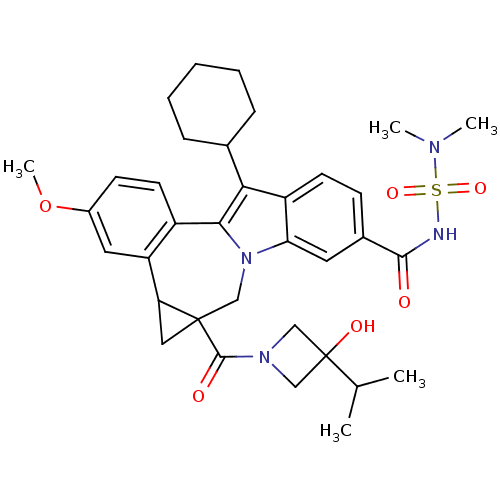 (CHEMBL3126855) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448514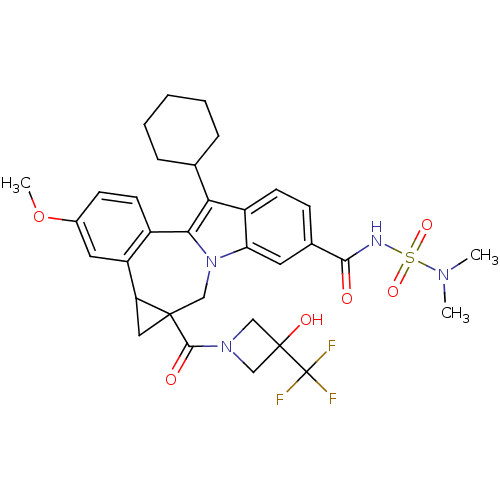 (CHEMBL3126854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448515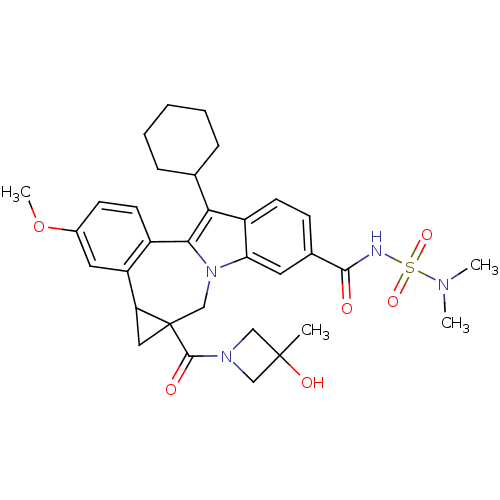 (CHEMBL3126853) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50448497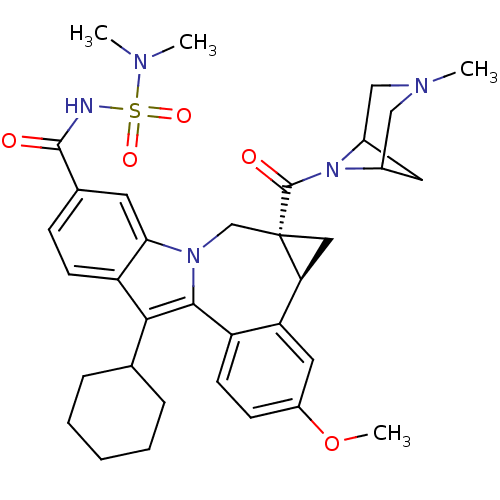 (CHEMBL3126834) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448496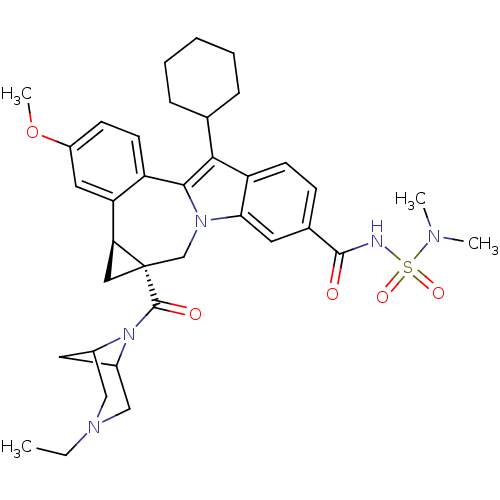 (CHEMBL3126835) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448495 (CHEMBL3126836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448497 (CHEMBL3126834) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50448495 (CHEMBL3126836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.30E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50448496 (CHEMBL3126835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448494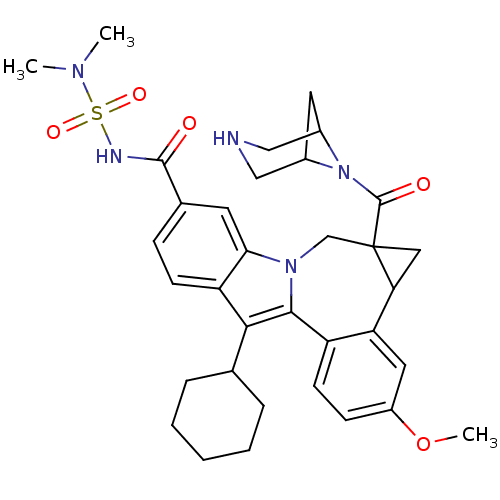 (CHEMBL3126657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50448497 (CHEMBL3126834) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448503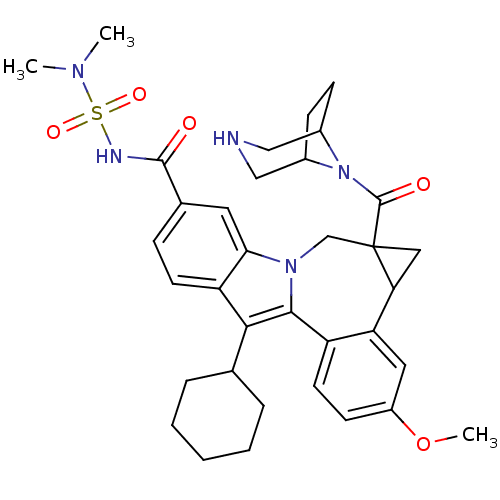 (CHEMBL3126837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448494 (CHEMBL3126657) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448505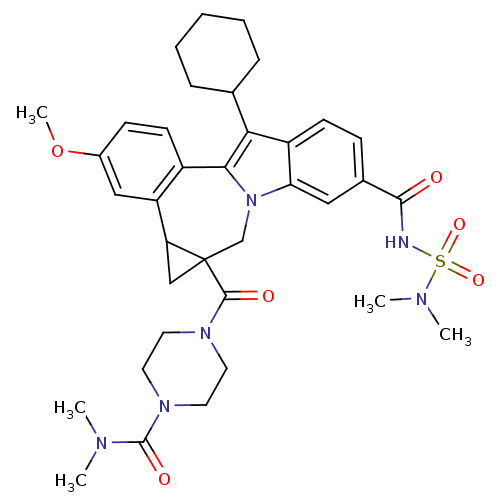 (CHEMBL3126656) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448495 (CHEMBL3126836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50448496 (CHEMBL3126835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448496 (CHEMBL3126835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448511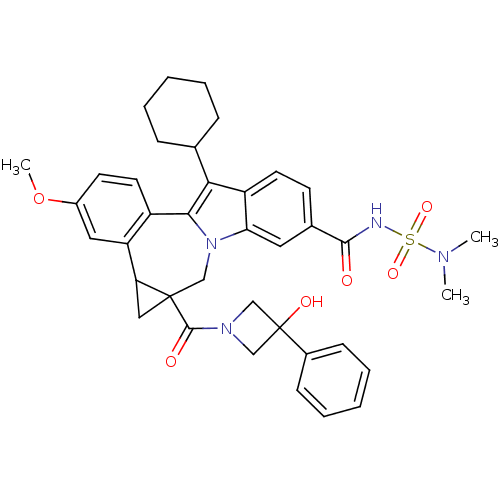 (CHEMBL3126857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448501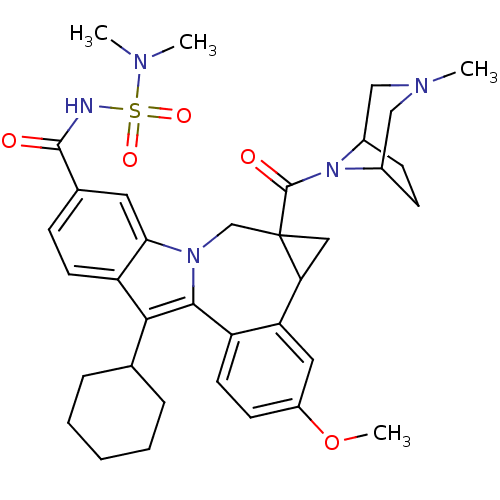 (CHEMBL3126839) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448495 (CHEMBL3126836) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448509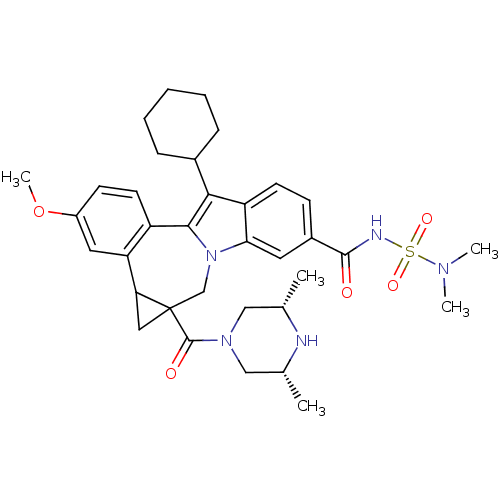 (CHEMBL3126859) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448517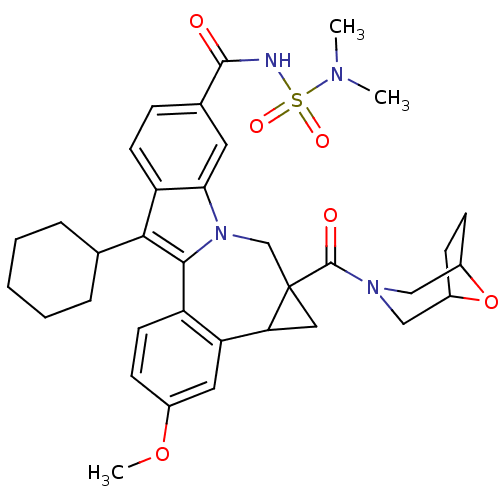 (CHEMBL3126851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448518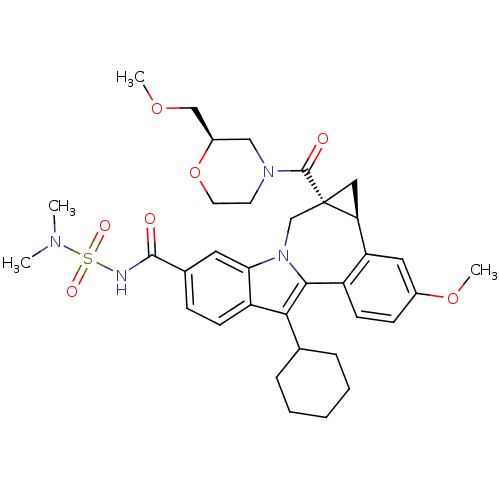 (CHEMBL3126849) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50448494 (CHEMBL3126657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448497 (CHEMBL3126834) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448498 (BMS-791325 | Beclabuvir) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448504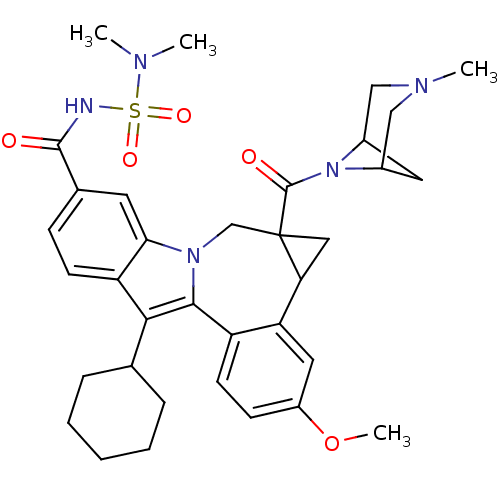 (CHEMBL3126658) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448506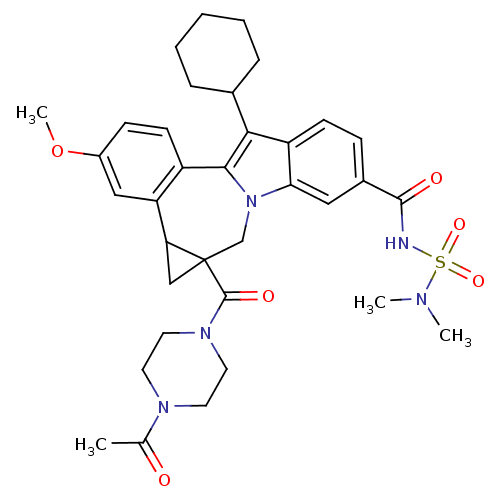 (CHEMBL3126655) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448516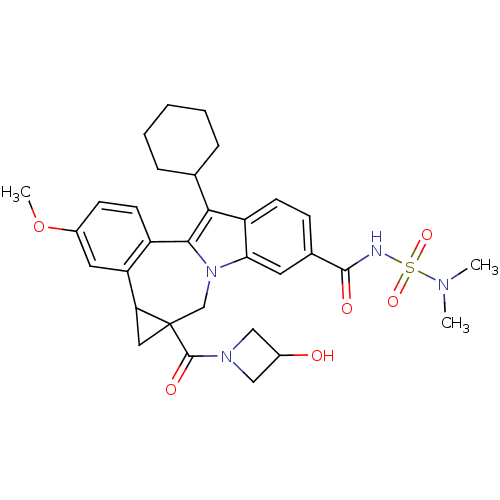 (CHEMBL3126852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50448494 (CHEMBL3126657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448497 (CHEMBL3126834) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448499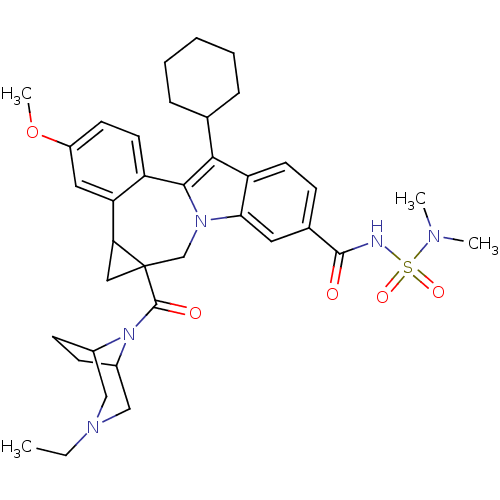 (CHEMBL3126841) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448502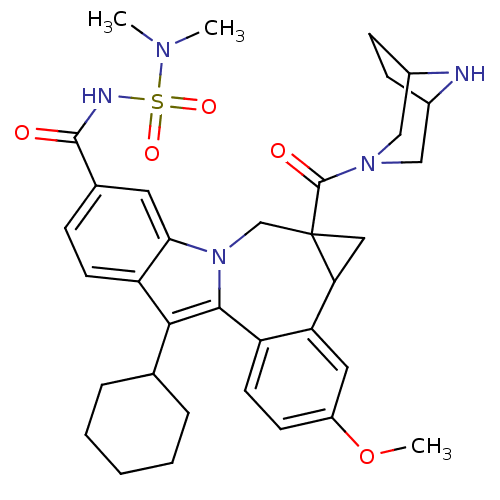 (CHEMBL3126838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448494 (CHEMBL3126657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448508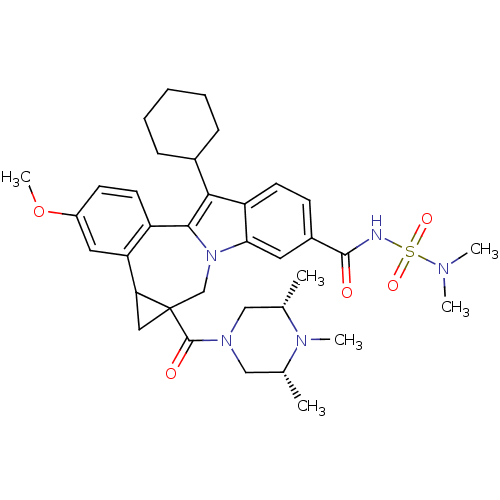 (CHEMBL3126860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448507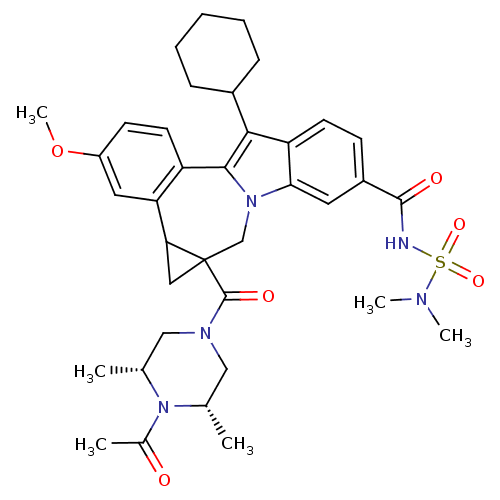 (CHEMBL3126861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50448512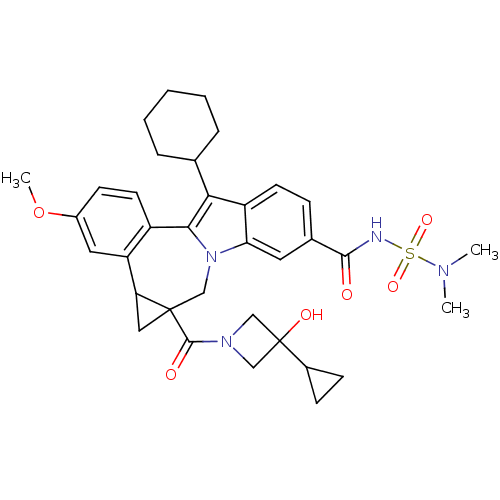 (CHEMBL3126856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in human HepG2 cells by luciferase reporter gene assay | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448496 (CHEMBL3126835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes after 30 mins in presence of NADPH | J Med Chem 57: 1855-79 (2014) Article DOI: 10.1021/jm4016894 BindingDB Entry DOI: 10.7270/Q2V989JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||