

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Homo sapiens (Human)) | BDBM50009200 (CHEMBL3238374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009201 (CHEMBL3238375) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009202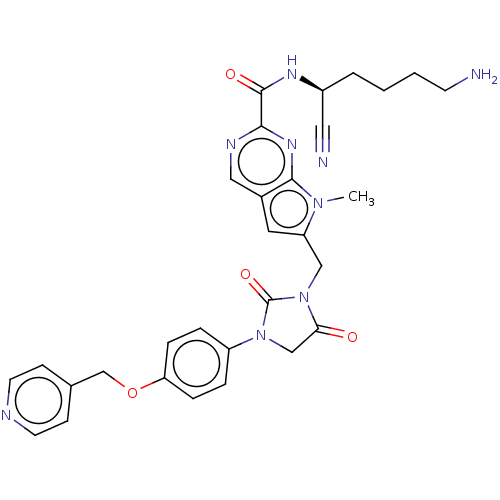 (CHEMBL3238376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009204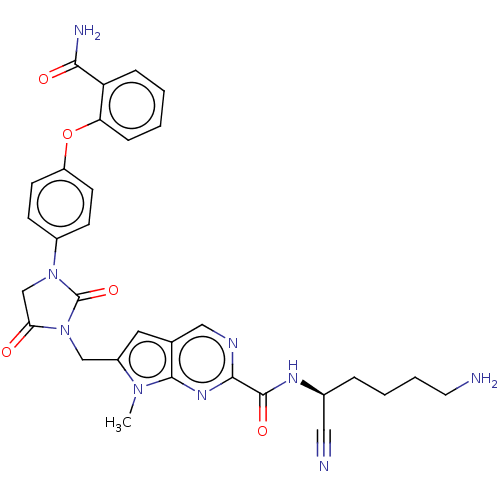 (CHEMBL3238378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009178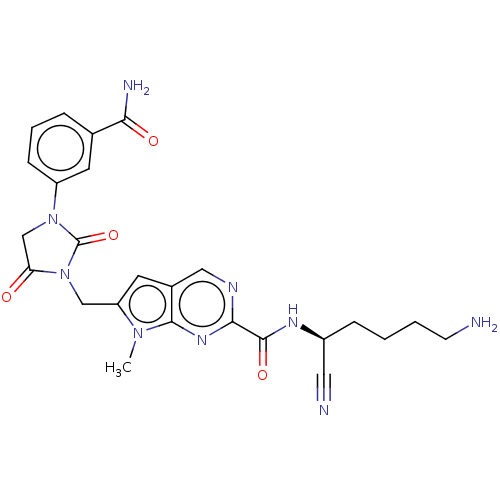 (CHEMBL3238369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009199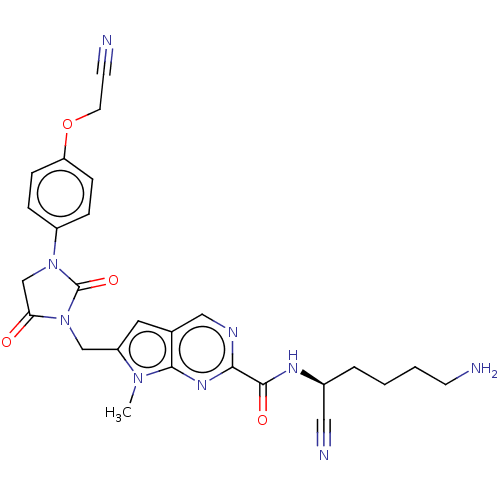 (CHEMBL3238373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009171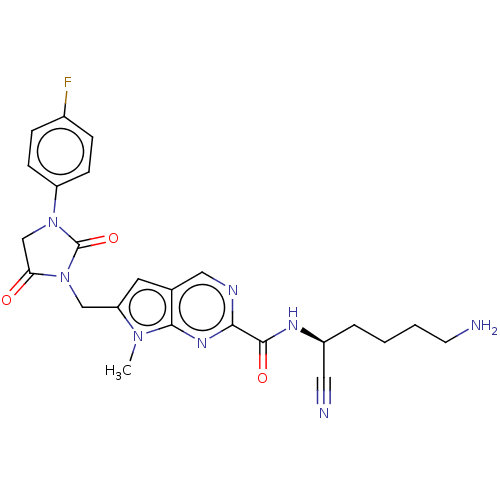 (CHEMBL3238362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009175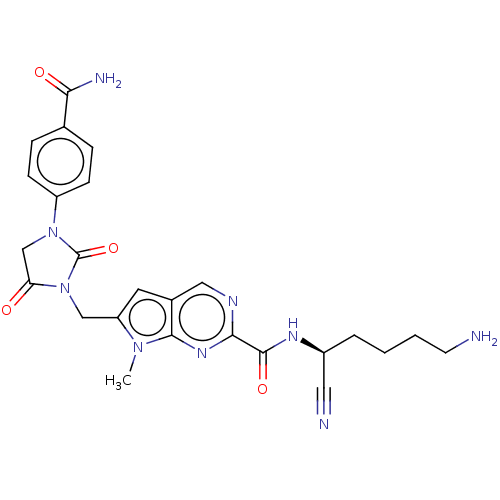 (CHEMBL3238366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009206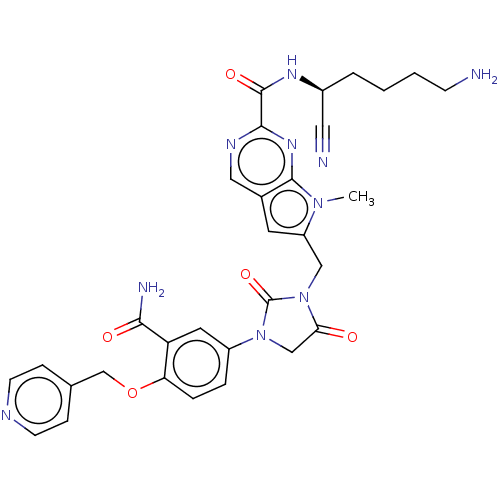 (CHEMBL3233150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009172 (CHEMBL3238363) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009209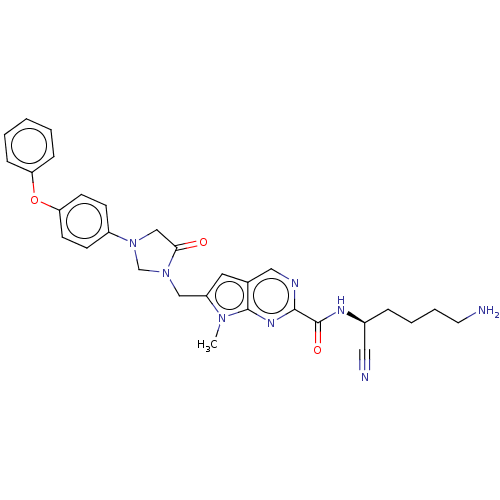 (CHEMBL3233152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009176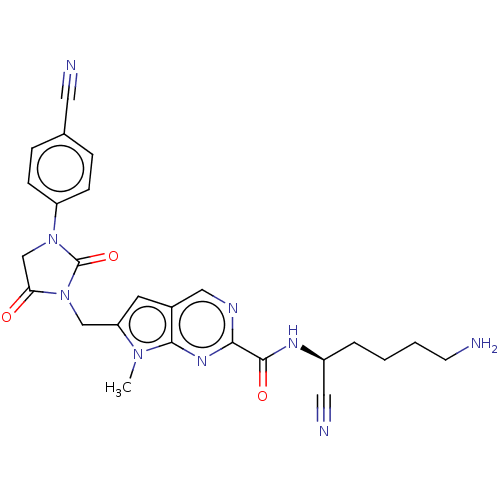 (CHEMBL3238367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009179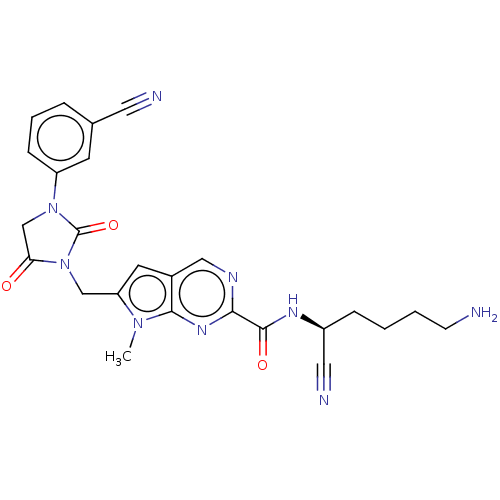 (CHEMBL3238370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009177 (CHEMBL3238368) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009203 (CHEMBL3238377) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009188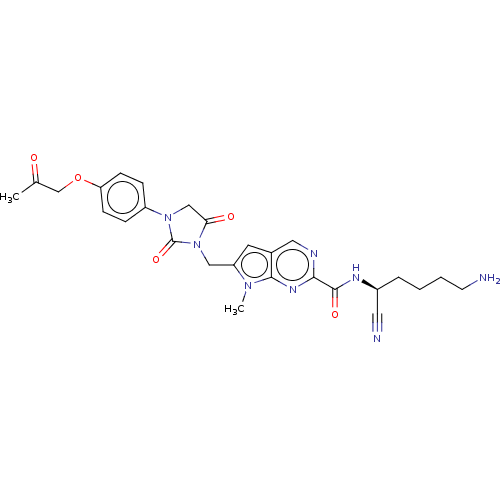 (CHEMBL3238371) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009209 (CHEMBL3233152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009174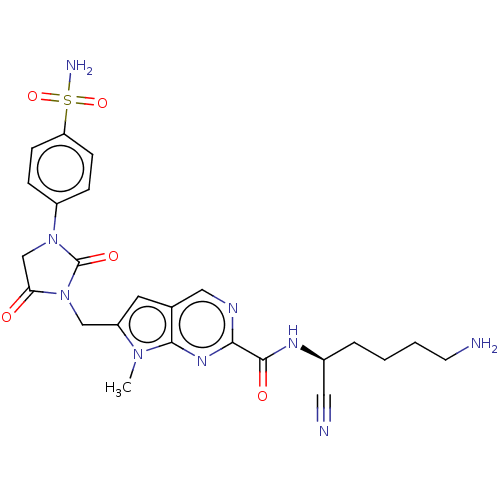 (CHEMBL3238365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009173 (CHEMBL3238364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009203 (CHEMBL3238377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009201 (CHEMBL3238375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009174 (CHEMBL3238365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009197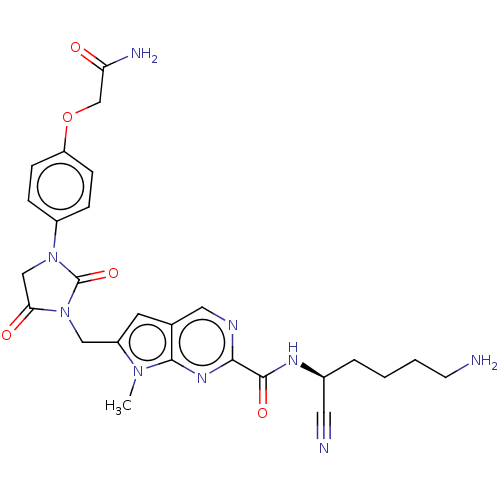 (CHEMBL3238372) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009200 (CHEMBL3238374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009208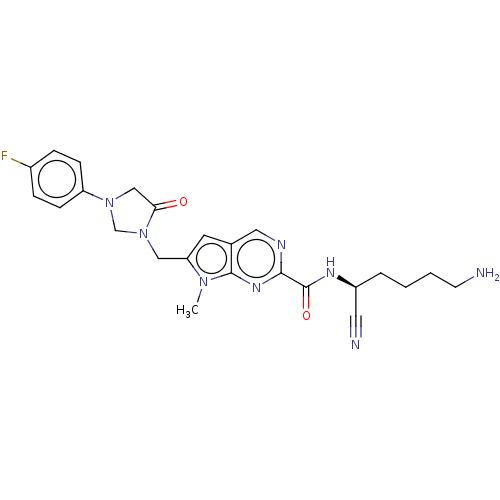 (CHEMBL3233151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009205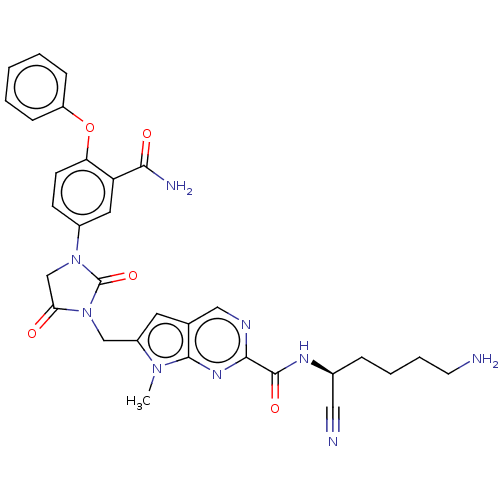 (CHEMBL3238379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009171 (CHEMBL3238362) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009210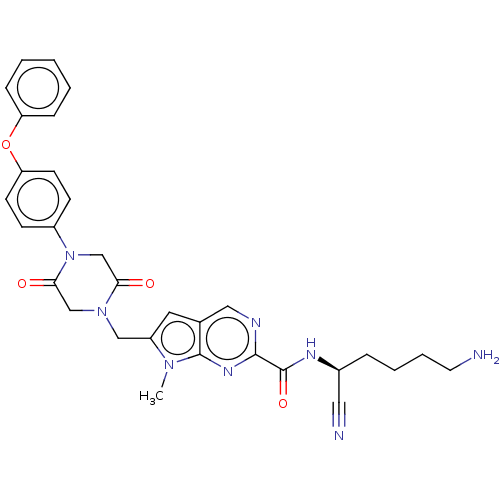 (CHEMBL3233153) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009202 (CHEMBL3238376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009205 (CHEMBL3238379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009173 (CHEMBL3238364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009178 (CHEMBL3238369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009199 (CHEMBL3238373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009170 (CHEMBL3238361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009179 (CHEMBL3238370) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009188 (CHEMBL3238371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009206 (CHEMBL3233150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50009170 (CHEMBL3238361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009175 (CHEMBL3238366) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009197 (CHEMBL3238372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009199 (CHEMBL3238373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009204 (CHEMBL3238378) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009176 (CHEMBL3238367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009177 (CHEMBL3238368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009197 (CHEMBL3238372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009204 (CHEMBL3238378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009176 (CHEMBL3238367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009208 (CHEMBL3233151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009210 (CHEMBL3233153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009174 (CHEMBL3238365) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009188 (CHEMBL3238371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009200 (CHEMBL3238374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009210 (CHEMBL3233153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009170 (CHEMBL3238361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009172 (CHEMBL3238363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009178 (CHEMBL3238369) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009171 (CHEMBL3238362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009202 (CHEMBL3238376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009206 (CHEMBL3233150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009208 (CHEMBL3233151) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009209 (CHEMBL3233152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009175 (CHEMBL3238366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009205 (CHEMBL3238379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009172 (CHEMBL3238363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009173 (CHEMBL3238364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009177 (CHEMBL3238368) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009201 (CHEMBL3238375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50009203 (CHEMBL3238377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50009179 (CHEMBL3238370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity | Bioorg Med Chem 22: 2339-52 (2014) Article DOI: 10.1016/j.bmc.2014.02.002 BindingDB Entry DOI: 10.7270/Q2KH0PVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||