

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/RI/5+/1957(H2N2)) recombinant neuraminidase using MUNANA as substrate after 30 mins | Bioorg Med Chem 22: 2236-43 (2014) Article DOI: 10.1016/j.bmc.2014.02.014 BindingDB Entry DOI: 10.7270/Q22F7PZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/RI/5+/1957(H2N2)) recombinant neuraminidase using MUNANA as substrate after 30 mins | Bioorg Med Chem 22: 2236-43 (2014) Article DOI: 10.1016/j.bmc.2014.02.014 BindingDB Entry DOI: 10.7270/Q22F7PZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50009296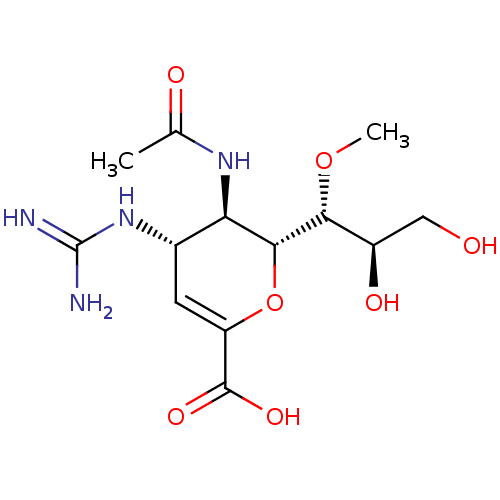 (LANINAMIVIR | R-125489) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/RI/5+/1957(H2N2)) recombinant neuraminidase using MUNANA as substrate after 30 mins | Bioorg Med Chem 22: 2236-43 (2014) Article DOI: 10.1016/j.bmc.2014.02.014 BindingDB Entry DOI: 10.7270/Q22F7PZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50009295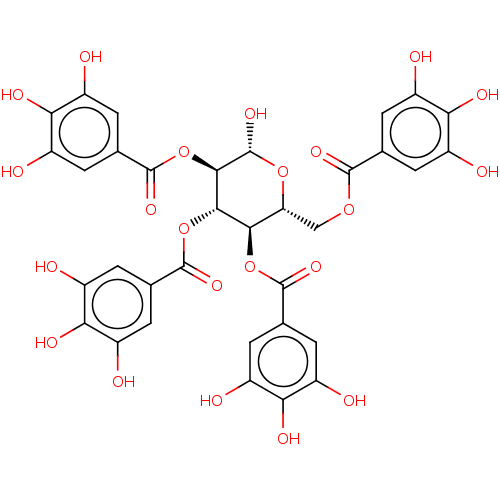 (CHEMBL500292) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/RI/5+/1957(H2N2)) recombinant neuraminidase using MUNANA as substrate after 30 mins | Bioorg Med Chem 22: 2236-43 (2014) Article DOI: 10.1016/j.bmc.2014.02.014 BindingDB Entry DOI: 10.7270/Q22F7PZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50241052 (1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/RI/5+/1957(H2N2)) recombinant neuraminidase using MUNANA as substrate after 30 mins | Bioorg Med Chem 22: 2236-43 (2014) Article DOI: 10.1016/j.bmc.2014.02.014 BindingDB Entry DOI: 10.7270/Q22F7PZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||