

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012188 (6BIO) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in fMLP-stimulated human monocytes assessed as reduction in HETE formation | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012188 (6BIO) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in LPS-stimulated human neutrophils assessed as reduction in HETE formation | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012188 (6BIO) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012188 (6BIO) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012188 (6BIO) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012184 (CHEMBL1092509) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012187 (INDIRUBIN 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012185 (CHEMBL3265230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012187 (INDIRUBIN 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012186 (CHEMBL3265231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012184 (CHEMBL1092509) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012186 (CHEMBL3265231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012185 (CHEMBL3265230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012185 (CHEMBL3265230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012186 (CHEMBL3265231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012187 (INDIRUBIN 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50012184 (CHEMBL1092509) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50132003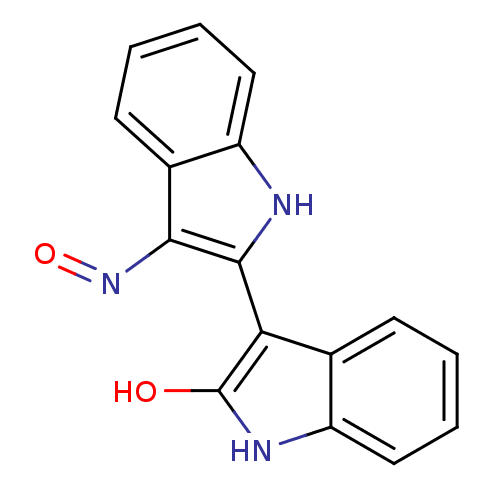 ((Z)-1H,1''H-[2,3'']Biindolylidene-3,2''-dione 3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho... | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50132003 ((Z)-1H,1''H-[2,3'']Biindolylidene-3,2''-dione 3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophore | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50132003 ((Z)-1H,1''H-[2,3'']Biindolylidene-3,2''-dione 3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to control | J Med Chem 57: 3715-23 (2014) Article DOI: 10.1021/jm401740w BindingDB Entry DOI: 10.7270/Q2NS0WGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||