

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50015606 (CHEMBL3260556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015614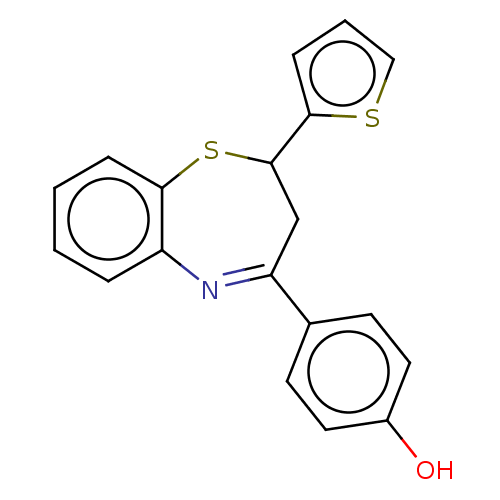 (CHEMBL3260200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50272839 (2-(Thien-2''-yl)-4-(3'-hydroxyphenyl)-2,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50272762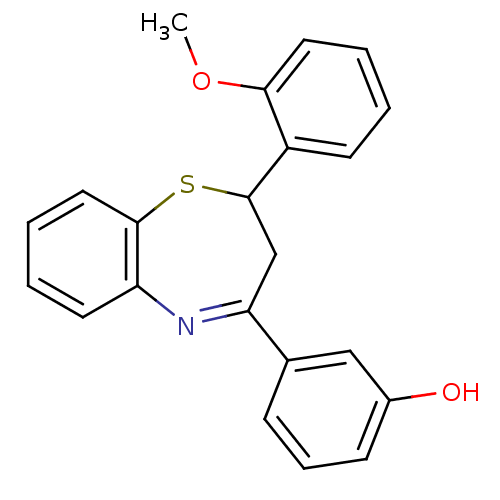 (2-(2''-Methoxyphenyl)-4-(3'-hydroxyphenyl)-2,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015615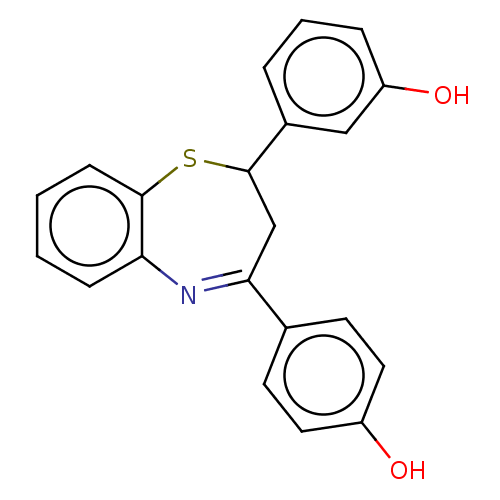 (CHEMBL3260201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015616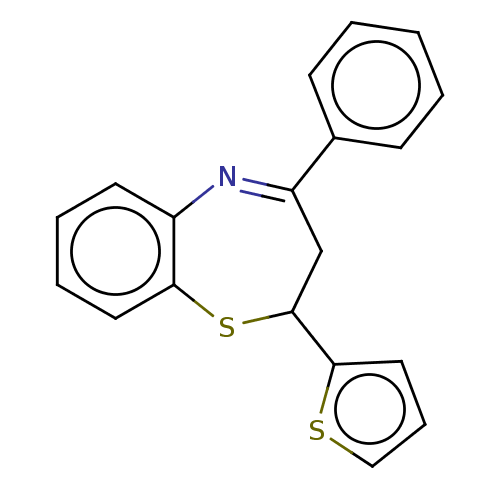 (CHEMBL3260553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015607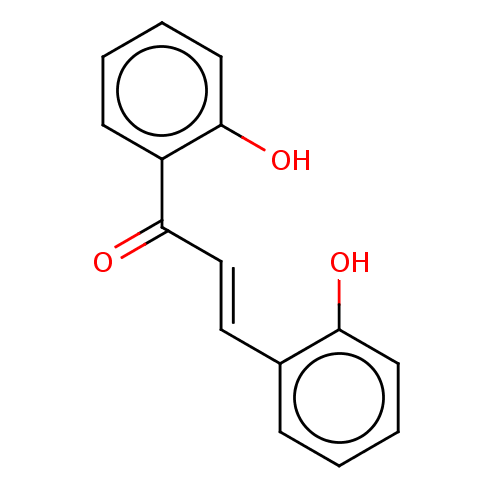 ((E)-1-(2-hydroxyphenyl)-3-(2-hydroxyphenyl)-2-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015628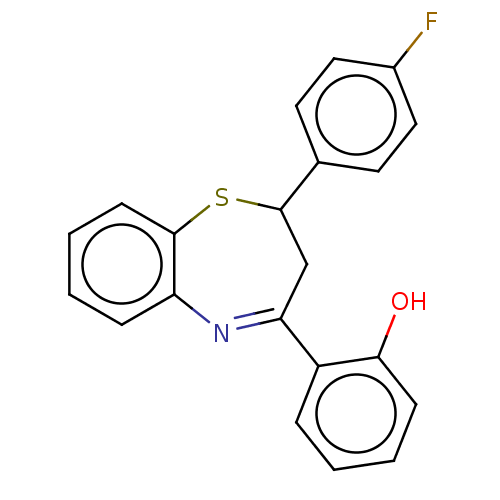 (CHEMBL2237295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015629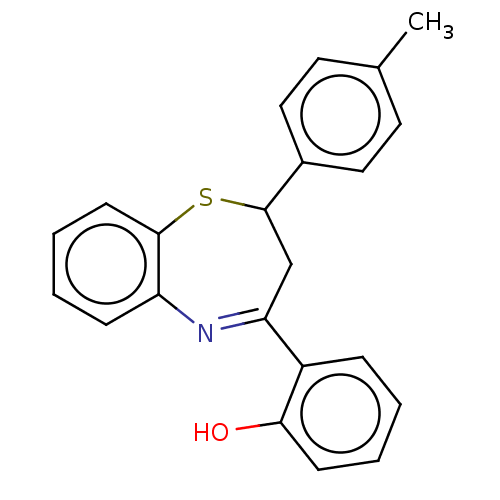 (CHEMBL2237298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015630 (CHEMBL3260554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015631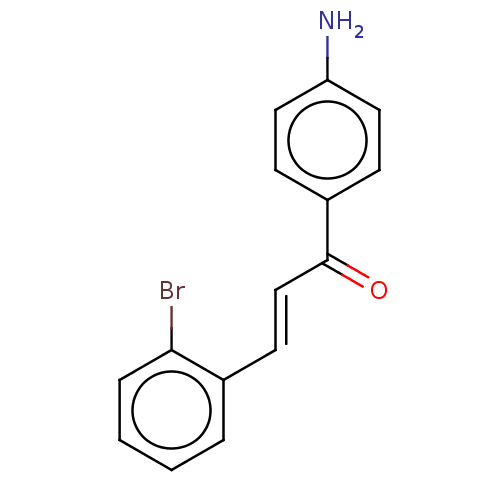 (CHEMBL2236848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM94616 ((E)-3-(2-methoxyphenyl)-1-phenyl-2-propen-1-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50272612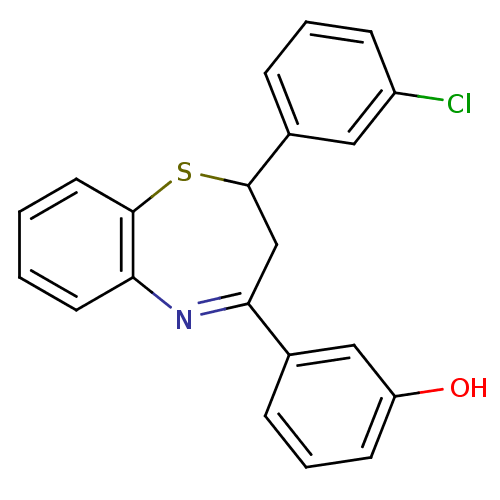 (2-(3''-Chlorophenyl)-4-(3'-hydroxyphenyl)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50440653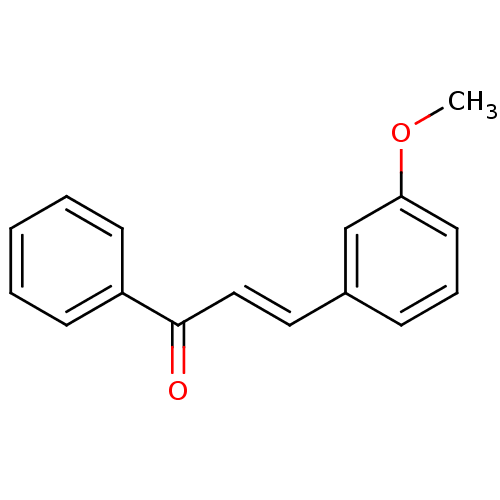 (CHEMBL318170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM33383 (phenylpropenone, 3-21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50272802 (2-(4''-N,N-Dimethylamino)-4-(3'-hydroxyphenyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015632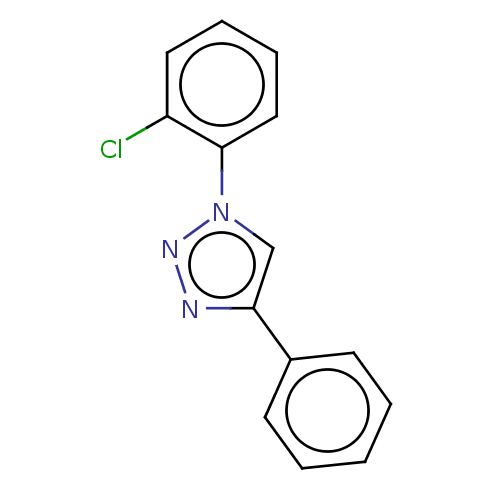 (CHEMBL3260177) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM86005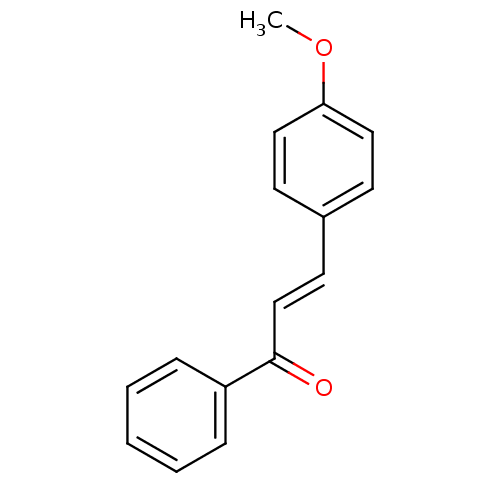 (Chalcone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015633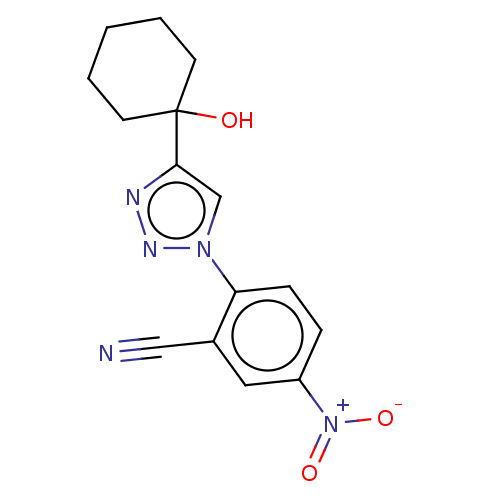 (CHEMBL3260180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015634 (CHEMBL3260182) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015635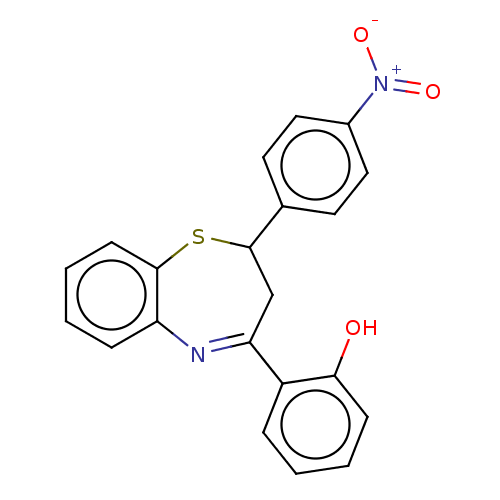 (CHEMBL3260555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015636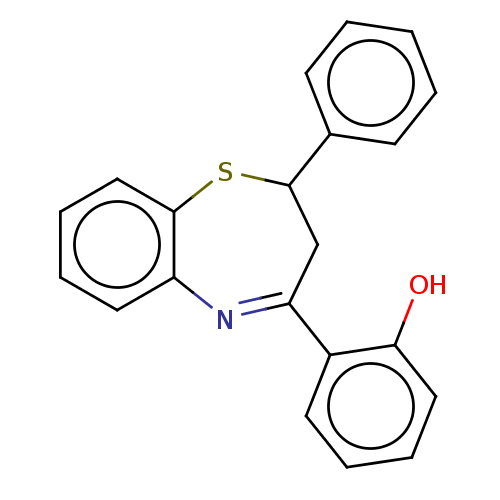 (CHEMBL2237293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015637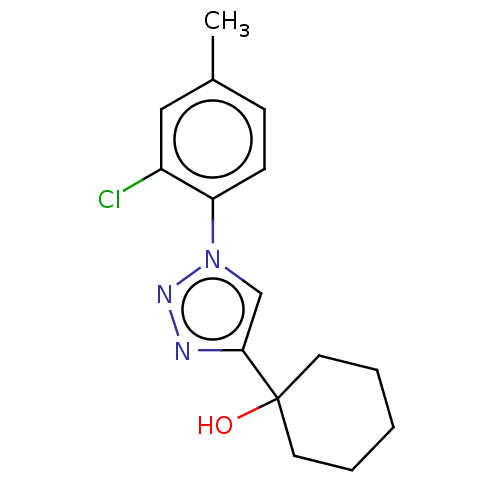 (CHEMBL3260186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50272801 (2-(4''-Methylphenyl)-4-(3'-hydroxyphenyl)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50272613 (2-(4''-Chlorophenyl)-4-(3'-hydroxyphenyl)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015638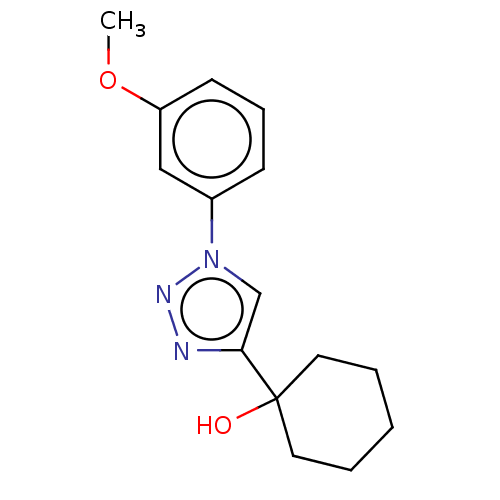 (CHEMBL3260190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM86007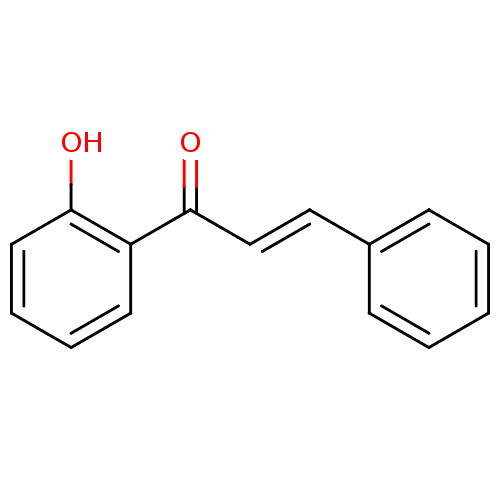 (Chalcone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM29143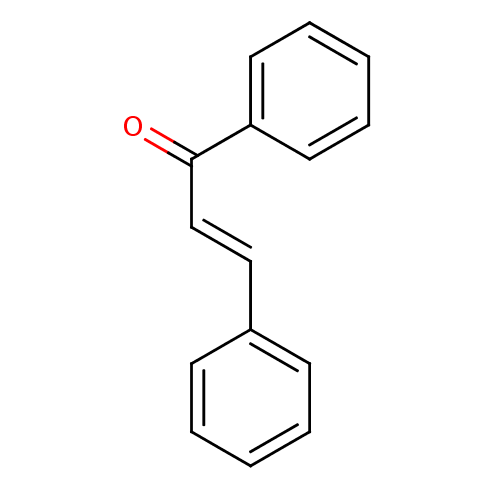 (CHEMBL7976 | Chalcone 1 | Chalcone, 13 | cid_63776...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM86002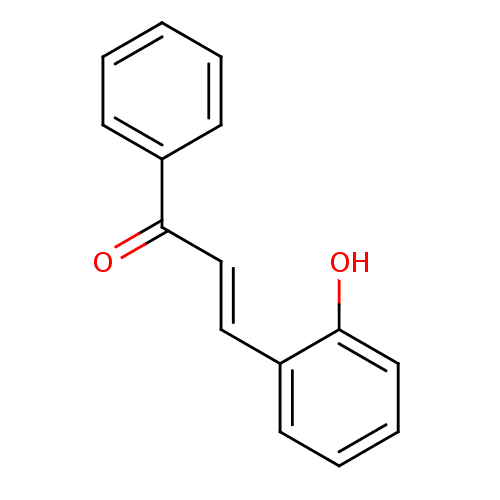 ((E)-1-phenyl-3-(2-hydroxyphenyl)-2-propen-1-one (8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015608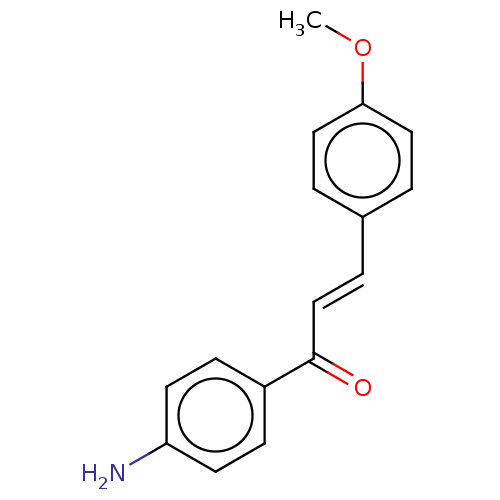 (CHEMBL469166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50273276 (2-(Phenyl)-4-(3'-hydroxyphenyl)-2,3-dihydro-1,5-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015609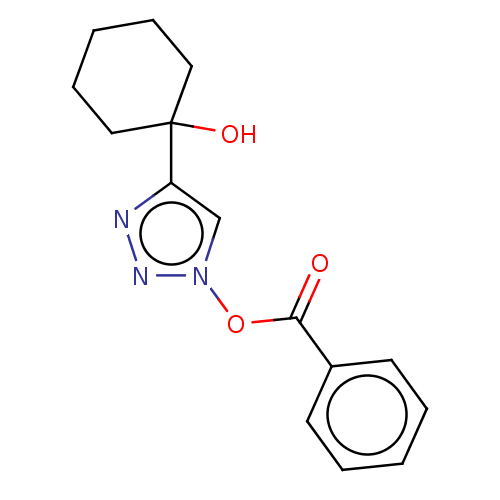 (CHEMBL3260181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015610 (CHEMBL1346776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015611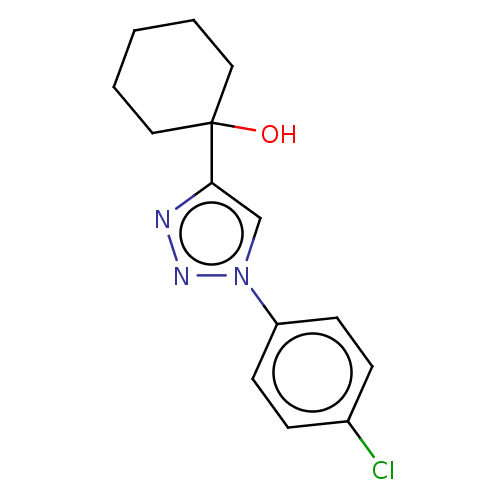 (CHEMBL3260183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015612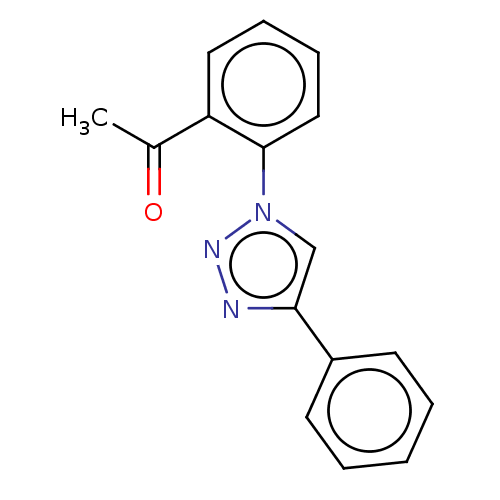 (CHEMBL3260192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015613 (CHEMBL3260189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015590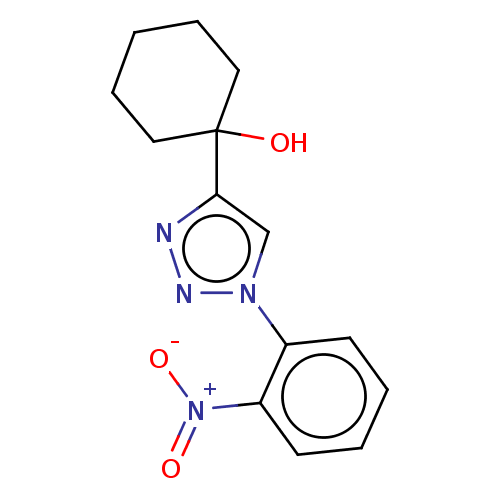 (CHEMBL3260193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015591 (CHEMBL3260194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015592 (CHEMBL3260178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015593 (CHEMBL3260195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015594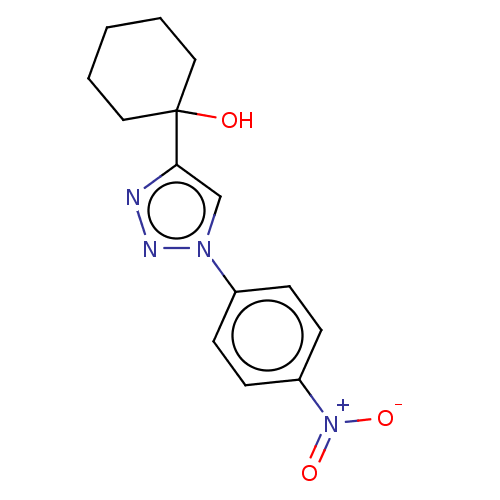 (CHEMBL3260185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015595 (CHEMBL3260175) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015596 (CHEMBL3260198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015597 (CHEMBL3260191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015598 (CHEMBL3260187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015599 (CHEMBL3260197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015600 (CHEMBL3260179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015601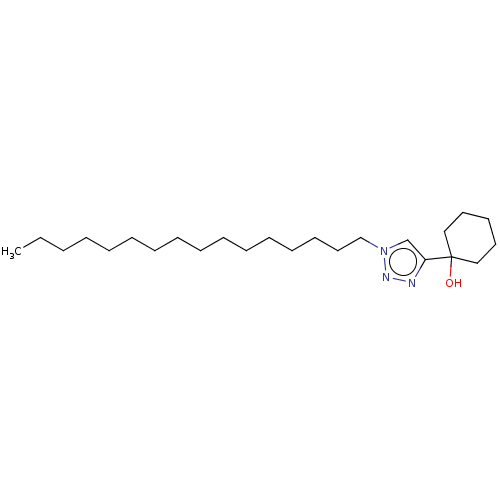 (CHEMBL3260174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015602 (CHEMBL3260188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015603 (CHEMBL3260196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015604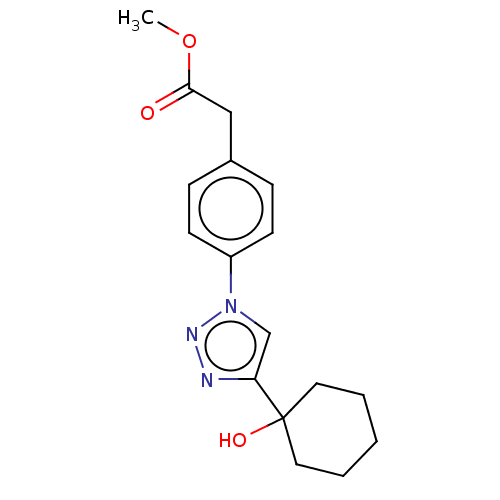 (CHEMBL3260184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50015605 (CHEMBL3260176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using BCh iodide as substrate preincubated for 15 mins prior to substrate addition measured after 10 mins by Ellm... | Eur J Med Chem 80: 228-42 (2014) Article DOI: 10.1016/j.ejmech.2014.04.018 BindingDB Entry DOI: 10.7270/Q2JM2C6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||