 Found 31 hits of Enzyme Inhibition Constant Data
Found 31 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50445061
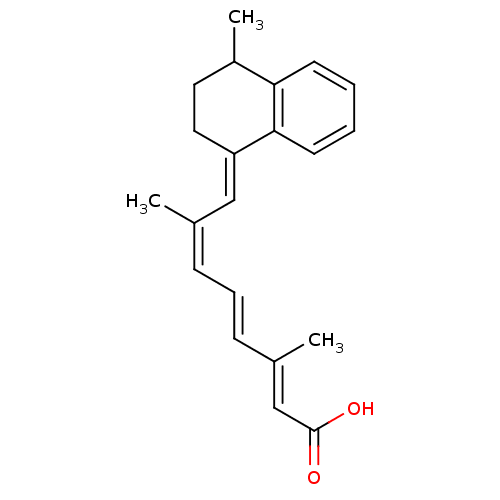
(CHEMBL3098772)Show SMILES CC1CC\C(=C/C(/C)=C\C=C\C(\C)=C\C(O)=O)c2ccccc12 Show InChI InChI=1S/C21H24O2/c1-15(7-6-8-16(2)14-21(22)23)13-18-12-11-17(3)19-9-4-5-10-20(18)19/h4-10,13-14,17H,11-12H2,1-3H3,(H,22,23)/b8-6+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM50018005

(CHEMBL3289660)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cccc(C)c12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-20(22)23)13-19-12-6-11-18-10-5-9-17(3)21(18)19/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,19-13+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018001
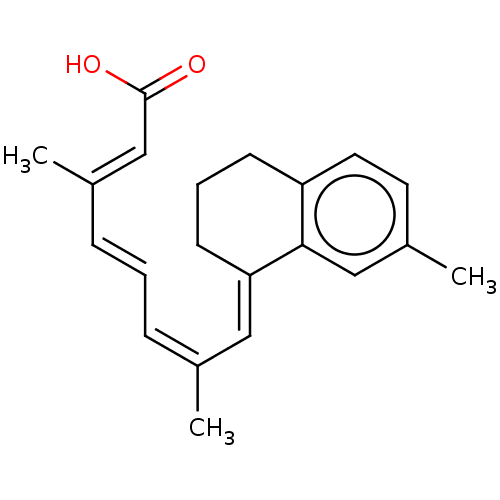
(CHEMBL3289659)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccc(C)cc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-19-9-5-8-18-11-10-17(3)13-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018004

(CHEMBL3289657)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2c(C)cccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-21(22)23)13-18-10-6-11-19-17(3)9-5-12-20(18)19/h4-5,7-9,12-14H,6,10-11H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50017994
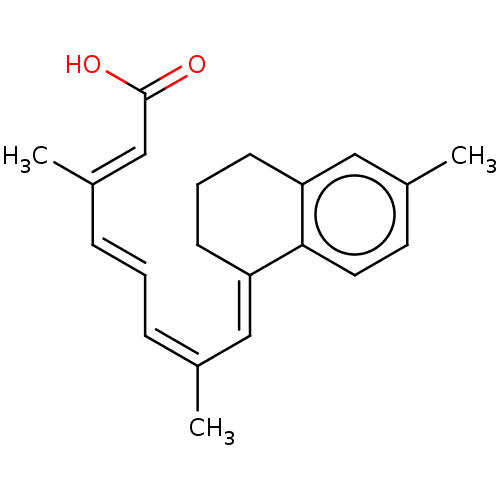
(CHEMBL3289658)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cc(C)ccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-18-8-5-9-19-13-17(3)10-11-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,18-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM50017994

(CHEMBL3289658)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cc(C)ccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-18-8-5-9-19-13-17(3)10-11-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,18-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50017994

(CHEMBL3289658)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cc(C)ccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-18-8-5-9-19-13-17(3)10-11-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,18-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018005

(CHEMBL3289660)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cccc(C)c12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-20(22)23)13-19-12-6-11-18-10-5-9-17(3)21(18)19/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50445062
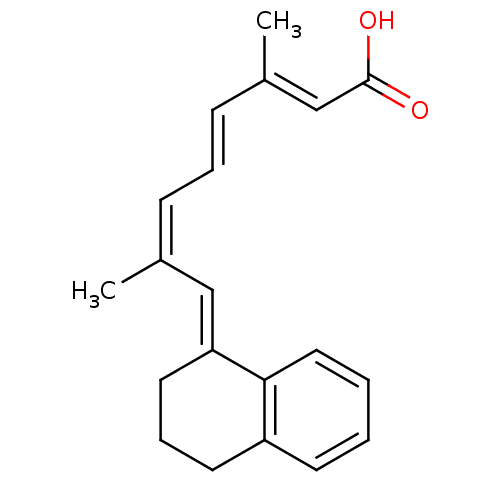
(CHEMBL3098771)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccccc12)=C\C(O)=O Show InChI InChI=1S/C20H22O2/c1-15(7-5-8-16(2)14-20(21)22)13-18-11-6-10-17-9-3-4-12-19(17)18/h3-5,7-9,12-14H,6,10-11H2,1-2H3,(H,21,22)/b8-5+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM50445062

(CHEMBL3098771)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccccc12)=C\C(O)=O Show InChI InChI=1S/C20H22O2/c1-15(7-5-8-16(2)14-20(21)22)13-18-11-6-10-17-9-3-4-12-19(17)18/h3-5,7-9,12-14H,6,10-11H2,1-2H3,(H,21,22)/b8-5+,15-7-,16-14+,18-13+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM50018004

(CHEMBL3289657)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2c(C)cccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-21(22)23)13-18-10-6-11-19-17(3)9-5-12-20(18)19/h4-5,7-9,12-14H,6,10-11H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,18-13+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM50018001

(CHEMBL3289659)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccc(C)cc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-19-9-5-8-18-11-10-17(3)13-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,19-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018001

(CHEMBL3289659)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccc(C)cc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-19-9-5-8-18-11-10-17(3)13-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50445062

(CHEMBL3098771)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccccc12)=C\C(O)=O Show InChI InChI=1S/C20H22O2/c1-15(7-5-8-16(2)14-20(21)22)13-18-11-6-10-17-9-3-4-12-19(17)18/h3-5,7-9,12-14H,6,10-11H2,1-2H3,(H,21,22)/b8-5+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50445060
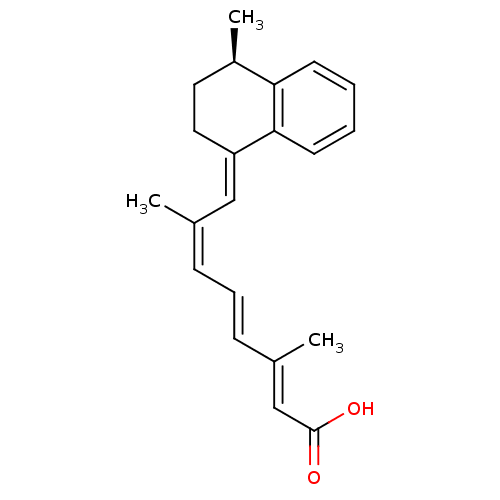
(CHEMBL3098773)Show SMILES C[C@@H]1CC\C(=C/C(/C)=C\C=C\C(\C)=C\C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C21H24O2/c1-15(7-6-8-16(2)14-21(22)23)13-18-12-11-17(3)19-9-4-5-10-20(18)19/h4-10,13-14,17H,11-12H2,1-3H3,(H,22,23)/b8-6+,15-7-,16-14+,18-13+/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018004

(CHEMBL3289657)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2c(C)cccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-21(22)23)13-18-10-6-11-19-17(3)9-5-12-20(18)19/h4-5,7-9,12-14H,6,10-11H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018004

(CHEMBL3289657)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2c(C)cccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-21(22)23)13-18-10-6-11-19-17(3)9-5-12-20(18)19/h4-5,7-9,12-14H,6,10-11H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50445060

(CHEMBL3098773)Show SMILES C[C@@H]1CC\C(=C/C(/C)=C\C=C\C(\C)=C\C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C21H24O2/c1-15(7-6-8-16(2)14-21(22)23)13-18-12-11-17(3)19-9-4-5-10-20(18)19/h4-10,13-14,17H,11-12H2,1-3H3,(H,22,23)/b8-6+,15-7-,16-14+,18-13+/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018005

(CHEMBL3289660)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cccc(C)c12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-20(22)23)13-19-12-6-11-18-10-5-9-17(3)21(18)19/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018001

(CHEMBL3289659)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccc(C)cc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-19-9-5-8-18-11-10-17(3)13-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50018005

(CHEMBL3289660)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cccc(C)c12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(7-4-8-16(2)14-20(22)23)13-19-12-6-11-18-10-5-9-17(3)21(18)19/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H,22,23)/b8-4+,15-7-,16-14+,19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.33E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50445062

(CHEMBL3098771)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2ccccc12)=C\C(O)=O Show InChI InChI=1S/C20H22O2/c1-15(7-5-8-16(2)14-20(21)22)13-18-11-6-10-17-9-3-4-12-19(17)18/h3-5,7-9,12-14H,6,10-11H2,1-2H3,(H,21,22)/b8-5+,15-7-,16-14+,18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is... |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50017994

(CHEMBL3289658)Show SMILES C/C(/C=C/C=C(/C)\C=C1/CCCc2cc(C)ccc12)=C\C(O)=O Show InChI InChI=1S/C21H24O2/c1-15(6-4-7-16(2)14-21(22)23)12-18-8-5-9-19-13-17(3)10-11-20(18)19/h4,6-7,10-14H,5,8-9H2,1-3H3,(H,22,23)/b7-4+,15-6-,16-14+,18-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(RAT) | BDBM50445060

(CHEMBL3098773)Show SMILES C[C@@H]1CC\C(=C/C(/C)=C\C=C\C(\C)=C\C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C21H24O2/c1-15(7-6-8-16(2)14-21(22)23)13-18-12-11-17(3)19-9-4-5-10-20(18)19/h4-10,13-14,17H,11-12H2,1-3H3,(H,22,23)/b8-6+,15-7-,16-14+,18-13+/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method |
J Med Chem 57: 5370-80 (2014)
Article DOI: 10.1021/jm5004792
BindingDB Entry DOI: 10.7270/Q2183821 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data